|
| Brian Hasinoff Medicinal Chemistry, College of Pharmacy University of Manitoba Winnipeg, Manitoba, Canada R3E 0T5 |
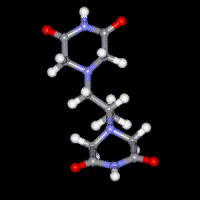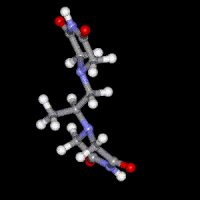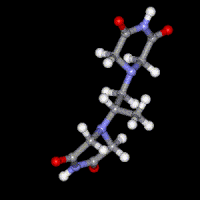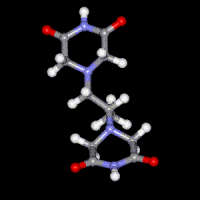
Dexrazoxane (ICRF-187)
Molecular Mechanisms of Cardiotoxic Anticancer Drugs and Targeting Topoisomerases
Program Overview
The various research projects in this laboratory aim at:
- an understanding of the mechanisms of oxygen and nitrogen free radical tissue damage and its prevention by antioxidant drugs
- the development of and an understanding of the mechanisms of action of anticancer drugs that target topoisomerase I and topoisomerase II
- the molecular mechanisms of cardiotoxic anticancer drugs
A number of previous studies have focused on the mechanism of action of the bisdioxopiperazine antioxidant drug dexrazoxane (ICRF-187) that is used clinically to prevent the dose-limiting cardiotoxicity caused by the antitumor anthracycline doxorubicin. Molecular modeling and docking are being used to design anticancer drugs that target topoisomerase I and topoisomerase II. More recently mechanisms of cardiotoxic anticancer kinase and proteasome inhibitors have been investigated.
Laboratory Capabilities
My laboratory is well equipped for a range of biological and biochemical studies. Equipment includes computer-controlled UV-vis double beam spectrophotometers, a spectrofluorimeter, fluorescence and absorbance plate readers, and computers for molecular modeling. The lab also has a Bruker EMX electron paramagnetic resonance (EPR) spectrometer for free radical, and nitric oxide and oxygen free radical spin trapping studies. The cell culture facility in the laboratory has laminar flow hoods, carbon dioxide incubators, and deconvolution epifluorescence and phase contrast microscopes. The lab also contains equipment for enzyme purification, cell component isolation, and gel electrophoresis equipment for Western blots and quantitative DNA gel assays.
Research Support
A grant from CIHR is currently supporting my research. Past research has been supported through a Tier I Canada Research Chair in Drug Development.
Some recent publications are listed here.
For additional information, please contact:Dr. Brian Hasinoff (Professor Emeritus)
College of Pharmacy
University of Manitoba
750 McDermot Avenue
Winnipeg, Manitoba R3E 0T5 Canada
Voice: (204) 474-8325 (office)
FAX: (204) 474-7617
E-mail: B_Hasinoff@UManitoba.ca
WWW home page: http://home.cc.umanitoba.ca/~hasinof/~hasinof.htm