
CHEM 1030
Carbon Chemistry in Nature & Society
CHEM 1030 Carbon Chemistry in Nature & Society
(3)
This course introduces organic molecules and illustrates the principles of organic chemistry with topics from cosmetics and personal care products, the petroleum industry, food preparation chemistry, polymers and plastics, poisons and biological toxins, and risk assessment. May not be used to fulfill chemistry requirements in a Chemistry Honours, Major, General, or Minor program. Not available to students who have previously obtained credit in, or are concurrently registered in, any 2000 level university chemistry course. Prerequisite: CHEM 1000 or 1300 or Chemistry 40S (or equivalent). Not to be held with the former 2.125.

Please read this important notice from the
Faculty of Science:
Registration advisory
Course Outline - 2009
Instructor:
Lectures:
- Tues. & Thurs. 2:30-3:45
PM
- 201 Armes
Recommended Textbooks:
- "Chemistry for Changing Times" 11th edition
J. W. Hill and D. K. Kolb; Prentice Hall Publishers
- Note: Much material from other sources
is used, and the book will not be followed closely. Reading a textbook is highly
recommended.
Evaluation:
- Mid-term test-1 TBA
--------------------------------------- 25%
- Mid-term test-2 TBA
--------------------------------------- 25%
- Final Exam Scheduled by
Student Records ------------ 50%
Final Exam Review
Students in the Faculty of Science are permitted to review their final exams before
the deadline for appealing final grades (Final Grade Appeal).
If you wish to view your final exam please go to the Department of Chemistry general
office (360 Parker Building), fill in an application form, and pay the $5.00
fee.
Academic Dishonesty: Please visit
the Faculty of Science web site
Cheating, Plagiarism etc.
The Frank & Donna Hruska
Prize:
-
My wife & I have set up a scholarship fund (The Frank & Donna Hruska
Prize) which awards seventy-five dollars to the student receiving
the top grade in CHEM 1030.
This award will appear on the winner's university
transcript.
TOPICS
I THE UBIQUITOUS CARBON ATOM: INTRODUCTION TO ORGANIC CHEMISTRY
Historical perspective: Wohler & the abandonment of the Vital Force Theory.
Bonding properties of carbon.
Why carbon forms so many molecules.
II DIAMOND, GRAPHITE & FULLERENES
Allotropes illustrate the profound effect that structure has on properties.
III HYDROCARBONS
A. ALKANES
- Linear alkanes: Methane, ethane, propane, etc.
- Structural & molecular formulae.
- Nomenclature: derivation from Greek & Latin; some examples such as methyl from methe (wine) + hyle (wood); methanol, the "wine of wood" derived by destructive distillation of wood.
- Branched alkanes
- Structural, or constitutional, isomers.
- Etymology: "isos" same; "meros", parts. Other "iso" words in science (isotherm, etc)
- Importance of branching: engine knocking & octane numbers.
- Cycloalkanes
- structure
- "Anting" behaviour of birds: flowers containing the cyclopropane derivative pyrethrum are applied to feathers & provide birds with this source of insecticide.
- Properties of Alkanes
- Physical properties: water solubility & density (reasons why petroleum floats on water); melting points & boiling points (essential for understanding perfumes).
- Chemical Properties: (i) Unreactive: origin of name "paraffin"; use as lubricants, sealants. (ii) Combustion: a source of heat; incomplete combustion, a source of the pollutant, CO (hockey arenas); explosions (Westray Mine disaster); decompression chambers. (iii) Halogenation: source of many industrial chemicals. (iv) Cracking: important to petroleum industry.
B. ALKENES:
- Structure & nomenclature of ethylene, propylene
- Geometric (cis-trans) isomerism.
- Biological importance of cis-trans isomerism
- Insect pheromones: recognition of one or the other isomer. Pine-bark beetle traps.
- Mechanism of vision; retinol, vitamin A, b-carotene.
- Physical properties: If more than one C=C bond, the molecules may be colored (b-carotene) or aromatic (b-selinene in oil of celery).
- Chemical reactions of alkenes.
- Combustion
- Addition reactions: (i) Hydrogenation: in the food industry (production of "Crisco" from vegetable oil). (ii) Halogenation: production of vinyl chloride for polyvinyl chloride plastics. (iii) Hydration: production of industrial alcohols.
- Polymerization: Brief introduction into polymer chemistry.
- Industrial importance: ethylene & its uses
C. ALKYNES
- Structures: Combustion of acetylene & the acetylene torch.
D. AROMATIC MOLECULES
- Brief description of structure & properties; a few structures. Fused-ring systems.
- Etymology: origin of "aromatic" & "benzene".
- Biologically important aromatic molecules: some amino acids, Vitamin B2, etc.
IV PETROLEUM INDUSTRY
A. IMPORTANCE & BRIEF HISTORY OF ITS USE
- Etymology: "petra" + "oleum".
- History: Dead Sea seepages. N. American Indians. Oil Springs, Ontario. JD Rockefeller.
- Importance
B. PETROLEUM
- Refining: Fractionation, cracking, and reforming.
C. GASOLINE
- Knocking. Octane rating. Leaded gas & its problems. Other octane enhancers–MTBE.
D. INTRODUCTION TO FUNCTIONAL GROUPS
- A 2nd look at the Periodic Table.
V OTHER CLASSES OF ORGANIC MOLECULES
A HALOGENATED HYDROCARBONS
- Synthesis from hydrocarbons by substitution & addition.
- Uses of haloalkanes: methylene chloride (decaffeinated coffee); chloroform (anesthetic); carbon tetrachloride (solvent); phosgene (war gas)
- CFCs.
- DDT: insecticide: World war II; WHO; malaria; Negative Effects; Peregrine falcon; Carson's "Silent Spring"; alternatives to DDT.
- Perfluorohydrocarbons: blood-substitutes.
B. ALCOHOLS
- General: structure & naming.
- Physical properties: H-bonding & its effect on boiling points; relevance in perfume industry.
- Methanol: synthesis & uses; toxicity.
- Ethanol: production for human consumption (fermentation) & industry (hydration of ethylene); uses.
- Isopropanol: Rubbing alcohol, preservative of zoological specimens. Precursor of nerve gases (Sarin, binary weapons, Aum Shinri Kyo cult in Japan, action on cholinesterase).
- Ethylene glycol: Synthesis. Use as automotive antifreeze, in manufacture of polyesters (Dacron in clothes, artificial blood vessels; Mylar for audio & video tapes).
- Glycerol:
- properties; importance emollient, humectant; soap.
- a biological antifreeze: hibernating frogs. Samuel Hearne in the NW Territories.
- Nitroglycerine: synthesis in 1847. Nobel, dynamite & Prizes. Building the CPR. Medical use (angina pectoris; the active agent is nitric oxide). Things that go bang can keep us alive!
- Phenols
- Uses as antiseptics, food preservatives (BHT); laxatives (phenolphthalein).
- Lister & history of antiseptics.
- Found in amino acids (tyrosine), marijuana, poison ivy.
- Antioxidant food additives: BHA & BHT, phenolic spices, vitamin E
- Picric acid: an explosive with a Canadian connection (Halifax explosion).
- Bombardier beetle: oxidation of hydroquinones as a defense mechanism
- Thiols: sulfur analogs of alcohols & phenols.
- skunks, rotten eggs, addition to natural gas, occurrence in hair (cysteine)
- Onions & garlic
- Important biochemical reaction of thiols: oxidation of thiols to disulfides & reduction of disulfides to thiols; curling of hair.
- Chemical Reactions of Alcohols
- Dehydration to ethers.
- Oxidation to aldehydes or ketones
- Breathalyzer test for intoxication.
C. ETHERS
- Properties (Chemically inert but flammable). Medical uses: Anesthetics.
D ALDEHYDES
- Some structures: synthesis by oxidation of alcohols.
- Formaldehyde
- uses (formalin, embalming); inactivation of virus for vaccines.
- UFFI: urea formaldehyde foam insulation.
- Methanol & blindness: conversion to formaldehyde
- Acetaldehyde
- Hangovers & alcoholism
- Treatment of alcoholism: the discovery of Antabuse illustrates the importance of Serendipity in science & medicine.
- 2-Methylundecanal: First synthetic "high note" in Chanel N°5; Marilyn Monroe.
- Vanilla: an interesting molecule with 3 functional groups (aldehyde, ether, phenol)
- Uses, source (orchid), brief history.
- Chemical composition, ripening of bean.
- artificial vanillin from lignin in wood.
- The unexpected in chemistry: vanilloid fragment found in capsaicin, the pungent principle of chilli pepper.
E. KETONES
- Acetone
- synthesis and uses .
- Steroid hormones with keto & aldehyde groups. RU486, a birth control drug.
- Acetone & the Formation of Israel: (i) illustrates impact of chemistry & biotechnology on politics. (ii) Chaim Weizmann; born Russia; organic chemist in England; first president of Israel. (iii) British need for acetone to prepare cordite for World War I. (iv) Weizmann's use of bacterial fermentation for acetone production. (v) Favorable view of Zionism by British leads to Balfour Declaration, etc.
- Diacetyl: ketone that gives butter its characteristic odor.
- Mating Pheromone of the red-sided garter snake
- The mating habits of Manitoba's world-renown reptile is controlled by a ketone.
- Snake behaviour & the vomeronasal organ that detects the pheromone.
F. CARBOXYLIC ACIDS
- Structures & general properties: aromatic & aliphatic; ionization.
- Formic acid
- origin of name (Latin formica = ant).
- Anting: Some birds rub their feathers with ants that produce formic acid (an insecticide), ridding them of lice, etc.
- Acetic acid
- Present in vinegar, sourdough bread.
- Uses in polymer industry (polyvinyl acetate, cellulose acetate); lead acetate in Grecian Formula.
- Propionic acid: Food preservative (inhibits mold growth). Naproxen (anti-inflammatory). Butyric: rancid butter. Body odor: contributions from carboxylic acids.
- Lactic Acid
- Etymology: Latin, lac = milk.
- Formed in active muscle; in brain during stroke; in heart suffering angina (Connection made here with nitroglycerin which alleviates the pain via nitric oxide.)
- Produced by "lactic acid bacteria" (Fermentation of dairy products: cheese, buttermilk, yogurt, sour cream) & other foods (sauerkraut, pickles, sourdough bread).
- Souring & curdling of milk: Little Miss Moffat and her curds & whey.
- Tooth decay: (i) oral bacteria (Streptococcus mutans) convert sugar to dextrans & plaque. (ii) Bacterial metabolism leads to lactic acid & to the dissolution of hydroxyapatite in enamel. (iii) Fluoride converts hydroxyapatite to fluorapatite, which is more resistant to acid.
- Miscellaneous: Slaughtering cattle, muscle cramps & biodegradable garbage bags.
G. ESTERS
- Examples of esters isolated from fruits & flowers. Chemical preparation.
- Waxes: Properties. Carnauba, ear wax, paraffin "wax".
- Fats & oils: Triacyglycerols. Hydrogenation of oils in margarine production. Soaps.
- Fat substitutes: Olestra: benefits and health risks.
- Aspirin (acetylsalicylic acid) & Oil of Wintergreen (methyl salicylate)
- Salicylic acid found in willow bark is both a phenol & aromatic carboxylic acid.
- Acetylating the phenol yields aspirin; esterifying the carboxyl group with methanol yields oil of wintergreen.
- Synthesis of aspirin & oil of wintergreen from petroleum feed stocks.
- Nitroglycerine: an ester of glycerol & an inorganic acid (nitric acid).
H. AMINES & AMIDES
- Amines: Structure and ionization. Important amines: B-vitamins, adrenaline, serotonin, cocaine, Prozac, opium, heroin.
- Amides: Structure. Important amides: phenacetin, xylocaine, proteins, nylon.
POLYMERS
A INTRODUCTION
- Etymology. Natural & synthetic.
B ADDITION POLYMERS
- Synthesis: High Density, Low Density Polyethylene.
- Polymers of ethylene derivatives
- Teflon: (i) History: serendipitous discovery at DuPont. (ii) First used during World War II for isolation of U-235. (iii) Commercial uses: non-stick cookware, space travel, artificial blood vessels, etc.
- Natural rubber: Charles Goodyear & vulcanization
C CONDENSATION POLYMERS
- Polyesters: structure and synthesis. Uses of PET (polyethylene terephthalate: Dacron).
- Polyamides: Structure & synthesis. Nylon & Wallace Carothers. Uses.
Diamond
|
Graphite
|
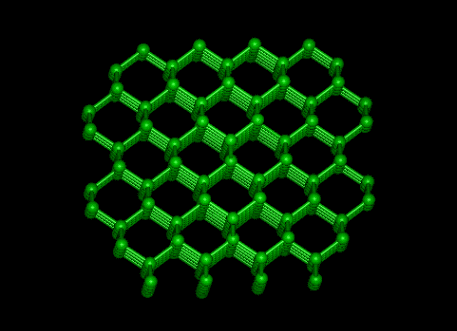 |
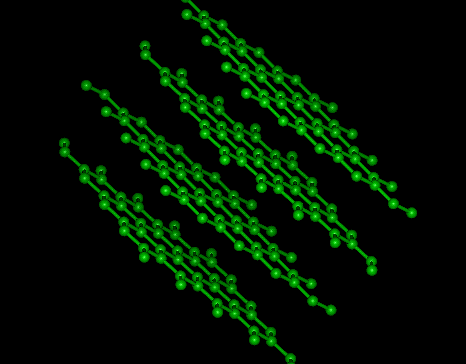 |
Fullerene
|
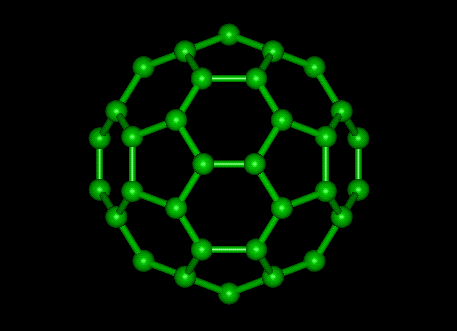 |
Useful Web Sites for CHEM 1030:
WebElementsTM Periodic Table
CHEMDEX
CIC
ACS
Return to the Chemistry Department Course Descriptions
Return to Dr. Hruska's Home Page
http://home.cc.umanitoba.ca/~joneil/CHEM 1030.Course.outline.htm
Maintained by J. O'Neil

Free JavaScripts provided
by The JavaScript Source







