Proteins
(Canadian Campbell 2nd ed -Concept 5.4)

Proteins have numerous important roles in the living organism. Proteins form
many structural features such as hair, hooves, and tendons. The elements in
proteins are carbon, hydrogen, oxygen,
nitrogen, and in some proteins, sulfur.
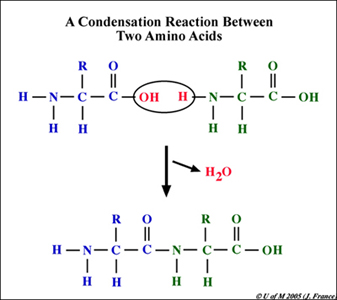
All proteins are polymers of amino acids covalently bonded in long chains that subsequently coil and fold into complex shapes that determine the function of the resulting protein molecule. The chemical bond that holds amino acids together is called a peptide bond. The bond forms between the functional groups of two amino acids. Specifically, a peptide bond occurs between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of its neighboring amino acid. Many amino acids linked together form a polypeptide. Proteins are polypeptides.
What is the common feature between glycosidic and peptide bond formation?
Enzymes are a particularly important class of proteins. They control the many chemical reactions that keep the cell alive. Acting as catalysts, the enzyme cause chemical reactions that would not occur in the absence of the enzymes or speed up reactions that would be very slow. Enzymes accomplish this effect by lowering the energy of activation of a biochemical reaction, which is the energy required to initiate the reaction. Specific substrates, or reactants, combine with enzymes at their active site(s), which induces biochemical reactions that result in products used by the cell. The activity of a specific enzyme is affected by many factors such as pH, temperature and inhibitors (substances that prevent normal action of the enzyme). A temperature and/or pH higher than what is normal for an enzyme will denature (alter the molecular shape of) the protein, thereby disabling the enzyme's normal activity. A pH lower than normal will have a similar effect. Enzymes are not denatured by lower temperatures, but the kinetic energy is lowered to a point where the rate of biochemical reactions is greatly reduced. Enzyme activity can be decreased by inhibitors that bind to the enzyme's active site. This prevents substrate molecules from binding and no chemical reaction occurs. Enzyme activity can also be decreased by inhibitors that bind to a site other than the active site of the enzyme. This renders the enzyme inoperative by altering its shape.
Exercise 5 - Effect of Temperature on Enzyme Activity
The enzyme you will be using, catechol oxidase, is found in plant tissues. As its name implies, catechol oxidase oxidizes (removes hydrogen from) the substrate catechol, also found in plants, and converts it to benzoquinone, which is brown in colour. This reaction explains the darkening of bruised fruit and vegetables. Catechol oxidase is released when a plant cell is damaged, and reacts with catechol to form benzoquinone. Under normal, unborken conditions, catechol and catechol oxidase do not come into contact with each other. Benzoquinone is toxic to bacteria and helps prevent decay in damaged plant tissue. Benzoquinone has a characteristic brown colour. In the lab we will use a potato as our source of catechol and catechol oxidase.
We will examine the rate at which catechol oxidase produces benzoquinone at different temperatures.
We used 5 test tubes set up as follows:
Tube 1
Tube 2
Tube 3
Tube 4
Tube 5
10 ml Water
10 ml Water
10 ml Water
10 ml Water
10 ml Water
-----
Cut-up Potato
Cut-up Potato
Cut-up Potato
Cut-up Potato
5 ml Catechol
5 ml Catechol
5 ml Catechol
5 ml Catechol
5 ml Catechol
Room Temp
100°C
Room Temp.
0°C
40°C
After 20 miniutes the tubes appeared as below:
Consider the following questions. For answers to the questions consult your lab manual, textbook and lecture notes.
![]() Back
to Lab Index
Back
to Lab Index ![]() Forward
to Next Page
Forward
to Next Page ![]() University
of Manitoba Home
University
of Manitoba Home