Protein Dynamics
Proteins are dynamic and their movements are key to biological function. We are interested in protein motions such as conformational changes and intrinsically disordered proteins (IDPs). IDPs and intrinsically disordered regions (IDRs) are common components of the proteome and provide numerous biochemical functions such as signaling, molecular transport, and even protection from dehydration. IDPs persist in a high energy state as an ensemble of secondary structure motifs or even exclusively as a random coil. When they bind an interaction partner, they can adopt a single conformation or remain largely disordered in a 'fuzzy complex.'
IDRs are often domain linkers or internal loop segments that undergo extensive conformational changes related to binding or enzymatic function.
Protein motions and disorder are essential to protein function, and we study the IDPs in the T6SS and in Streptococcus.
Proteins are dynamic and their movements are key to biological function. We are interested in protein motions such as conformational changes and intrinsically disordered proteins (IDPs). IDPs and intrinsically disordered regions (IDRs) are common components of the proteome and provide numerous biochemical functions such as signaling, molecular transport, and even protection from dehydration. IDPs persist in a high energy state as an ensemble of secondary structure motifs or even exclusively as a random coil. When they bind an interaction partner, they can adopt a single conformation or remain largely disordered in a 'fuzzy complex.'
IDRs are often domain linkers or internal loop segments that undergo extensive conformational changes related to binding or enzymatic function.
Protein motions and disorder are essential to protein function, and we study the IDPs in the T6SS and in Streptococcus.
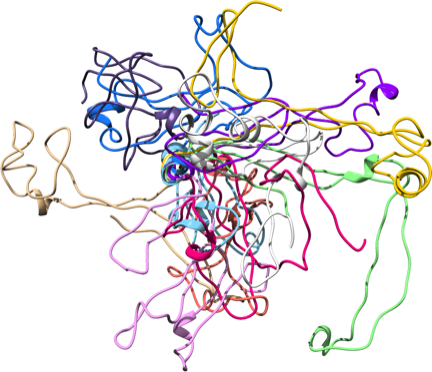
Ensemble of Bd0108, an IDP that helps regulate a critical type IV pilus of the predatory bacteria Bdellovibrio bacteriovorus. Calculated using NMR chemical shift data and CS-Rosetta.
We collaborate with Mazdak Khajehpour to study protein folding and dynamics and with Andrew Lovering to study dynamics of Bdellovibrio bacteriovorus proteins.
