The Molecules of Life: Biochemistry
Lipids
(Canadian Campbell 2nd ed -5.3)
 Lipids
are a varied group of molecules most of which are insoluble in water. Like carbohydrates,
carbon, hydrogen, and oxygen
are the principal elements of lipids although the oxygen content is much reduced.
Fats, phospholipids, steroids, carotenoids, and waxes are lipids. Fats are the
most abundant group of lipids in the biological world. Fats are composed of
three fatty acid molecules bonded to a molecule of the alcohol
glycerol. Fats have at least twice the energy storing capacity
per unit weight as carbohydrates do. Cholesterol is an important steroid that
is a part of some hormones. Steroids differ from most other lipids by virtue
of the structure of steroids consisting of carbon rings instead of chains.
Lipids
are a varied group of molecules most of which are insoluble in water. Like carbohydrates,
carbon, hydrogen, and oxygen
are the principal elements of lipids although the oxygen content is much reduced.
Fats, phospholipids, steroids, carotenoids, and waxes are lipids. Fats are the
most abundant group of lipids in the biological world. Fats are composed of
three fatty acid molecules bonded to a molecule of the alcohol
glycerol. Fats have at least twice the energy storing capacity
per unit weight as carbohydrates do. Cholesterol is an important steroid that
is a part of some hormones. Steroids differ from most other lipids by virtue
of the structure of steroids consisting of carbon rings instead of chains.
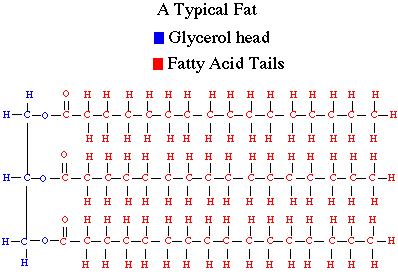
One of the biological important characteristics of fats, and lipids in general,
is their insolubility in water. Lipids are made of long chains of hydrocarbons
with relatively little oxygen (see figure to right). As a result of this, they
tend to be non-polar, meaning they do not dissolve in polar solvents such as
water. Today we are going to demonstrate this by dissolving a fat (vegetable
oil) into water and ether. The fat we will use, like that shown in the adjacent
figure, is a typical fat with three fatty acid chains. Another group of important
lipids are the phospholipids.
Phospholipids differ from fats in only having two fatty acid chains
and a polar head rather than a glycerol head. The insolubility
in water (hydrophobic) of the fatty acid tails and the solubility
of the polar head (hydrophilic) is important in the functioning
of the phospholipid membrane of cells.
Exercise 4 - Solubility
of Fats
Click
Here to view a tube containing 5 ml of vegetable oil
and 5 ml of water.
Blue dye was added to the water for increased visibility.
Consider the following questions. For answers to the questions consult your
lab manual, textbook and lecture notes. If you are still unsure consult with
one of the instructors.
- Do the oil and water mix?
- What can you conclude about the polarity of the oil if you know that water
is polar?
- The fat in the tube is a plant triglycerol (vegetable oil). Why is it a
liquid at room temperature?
- Would you expect an animal triglycerol to be solid or liquid at room temperature?
Why?
Click
Here to view the same tube with 5 ml of soap solution
added to the mix.
Consider the following questions. For answers to the questions consult your
lab manual, textbook and lecture notes. If you are still unsure consult with
one of the instructors.
- Did the oil and water mix when you added the soap?
- What did the soap do to the fat?
- Can you think about process and locations were a compounds like the soap
would be important to an animal?
Click
Here to view a tube containing 10 ml of vegetable oil
and 10 ml of ether.
Consider the following questions. For answers to the questions consult your
lab manual, textbook and lecture notes. If you are still unsure consult with
one of the instructors.
- Did the oil and ether mix?
- Now that you know that oil is non polar what can you conclude about the polarity of the ether?
- Fats do not dissolve in water but phospholipids used to form membranes can disperse in water to form micelles. Why?
First published Sept 95: Modified June 2020
Copyright © Michael Shaw 2019 (Images and Text)
 Back
to Lab Index
Back
to Lab Index  Forward
to Next Page
Forward
to Next Page  University
of Manitoba Home
University
of Manitoba Home
 Lipids
are a varied group of molecules most of which are insoluble in water. Like carbohydrates,
carbon, hydrogen, and oxygen
are the principal elements of lipids although the oxygen content is much reduced.
Fats, phospholipids, steroids, carotenoids, and waxes are lipids. Fats are the
most abundant group of lipids in the biological world. Fats are composed of
three fatty acid molecules bonded to a molecule of the alcohol
glycerol. Fats have at least twice the energy storing capacity
per unit weight as carbohydrates do. Cholesterol is an important steroid that
is a part of some hormones. Steroids differ from most other lipids by virtue
of the structure of steroids consisting of carbon rings instead of chains.
Lipids
are a varied group of molecules most of which are insoluble in water. Like carbohydrates,
carbon, hydrogen, and oxygen
are the principal elements of lipids although the oxygen content is much reduced.
Fats, phospholipids, steroids, carotenoids, and waxes are lipids. Fats are the
most abundant group of lipids in the biological world. Fats are composed of
three fatty acid molecules bonded to a molecule of the alcohol
glycerol. Fats have at least twice the energy storing capacity
per unit weight as carbohydrates do. Cholesterol is an important steroid that
is a part of some hormones. Steroids differ from most other lipids by virtue
of the structure of steroids consisting of carbon rings instead of chains.