


Representative Research Projects and Publications (full list of publications is available here)
1. Organometallic porphyrins and their analogues
This research project is aimed at the development of new organometallic porphyrins and analogues and understanding of their electron-transfer and light-harvesting properties. In particular, we are interested in: (i) the fundamental understanding and improvement of a basic knowledge of structure, reactivity, and electronic interactions in complex redox-active porphyrinoid-based arrays, (ii) the search for aromatic macrocycles useful for applications in molecular electronics, light-harvesting, catalysis, and photocatalysis; (iii) the creation of new methodologies for preparation of porphyrins and their analogues with useful properties.
For instance, we have prepared and characterized a large number of metal-free and transition-metal ferrocenyl-containing porphyrins in which organometallic substituents are directly connected to the porphyrin core. Because of very unusual long-range metal-metal coupling between organometallic substituents, each ferrocene group has a different oxidation potential in non-polar solvents and non-coordinating electrolytes. Such properties are useful for application in molecular electronics and in particular high-density, low energy consumption random-access memory (RAM) electronics:
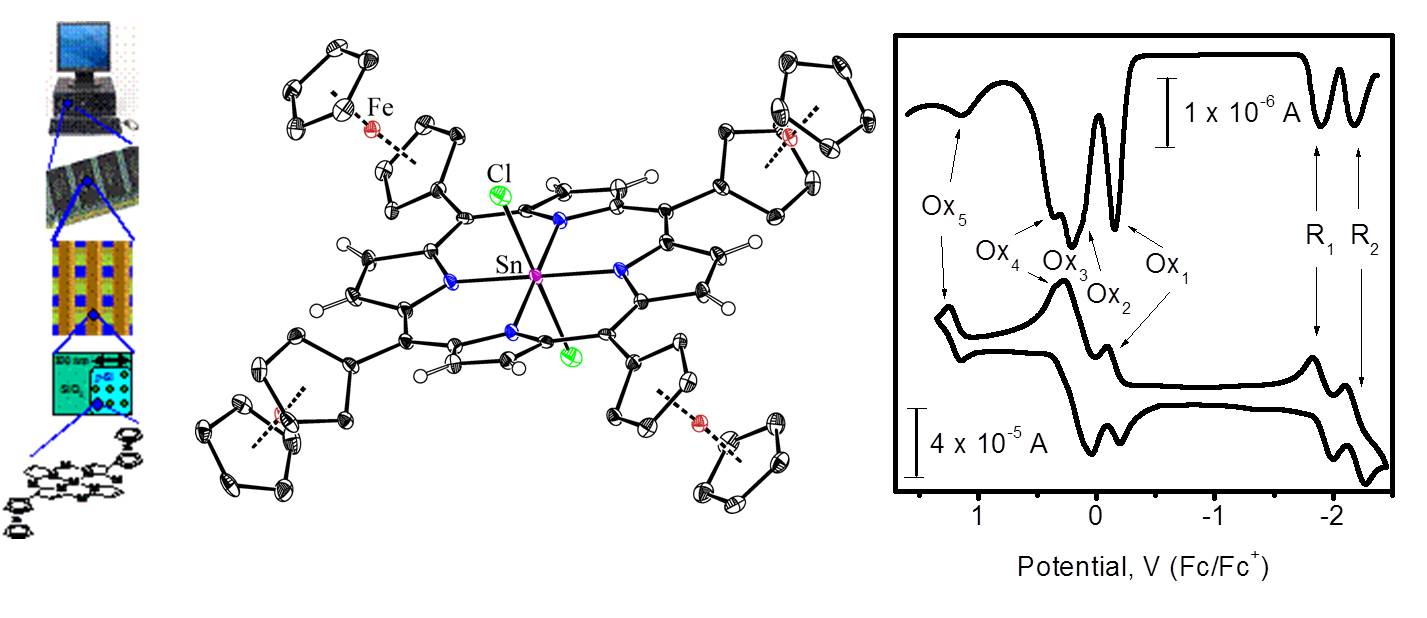
Representative publications: Nemykin, V. N.; Chen, P.; Solntsev, P. V.; Purchel, A. A.; Kadish, K. M. “Characterization of the unusual metal-free, zinc, chloroindium, and ferrocenylindium 5,10,15,20-tetraferrocenylporphyrin anion-radicals by spectroelectrochemical, DFT, and TDDFT approaches”. J. Porphyrins Phthalocyanines 2012 , 16 , 793-801. Solntsev, P. V.; Neisen, B. D.; Sabin, J. R.; Gerasimchuk, N. N.; Nemykin, V. N. “Synthesis, characterization, X-ray structure, and mixed-valence states of trans -dichlorotin(IV)-5,10,15,20-tetraferrocenylporphyrin”. J. Porphyrins Phthalocyanines, 2011 , 15 , 612-621. Rohde, G. T.; Sabin, J. R.; Barrett, C. D.; Nemykin, V. N. “Long-range metal–metal coupling in transition-metal 5,10,15,20-tetraferrocenylporphyrins”. New J. Chem. , 2011 , 35 , 1440-1448. Nemykin, V. N.; Rohde, G. T.; Barrett, C. D.; Hadt, R. G.; Sabin, J. R.; Reina, G.; Galloni, P.; Floris, B. "Long-range electronic communication in free-base meso -poly(ferrocenyl)-containing porphyrins". Inorg. Chem. 2010 , 49 , 7497-7509. Nemykin, V. N.; Rohde, G. T.; Barrett, C. D.; Hadt, R. G.; Bizzarri, C.; Galloni, P.; Floris, B.; Nowik, I.; Herber, R. H.; Marrani, A. G.; Zanoni, R.; Loim, N. M. " Electron-Transfer Processes in Metal-Free Tetraferrocenylporphyrin. Understanding Internal Interactions To Access Mixed-Valence States Potentially Useful for Quantum Cellular Automata". J. Am. Chem. Soc. 2009 , 131 , 14969-14978.
When immobilized on the gold surface, in polar solvents, tetraferrocenylporphyrins can be used in four-electrons photocatalytic reactions. For instance, they can be used in photocatalytic reduction of oxygen:
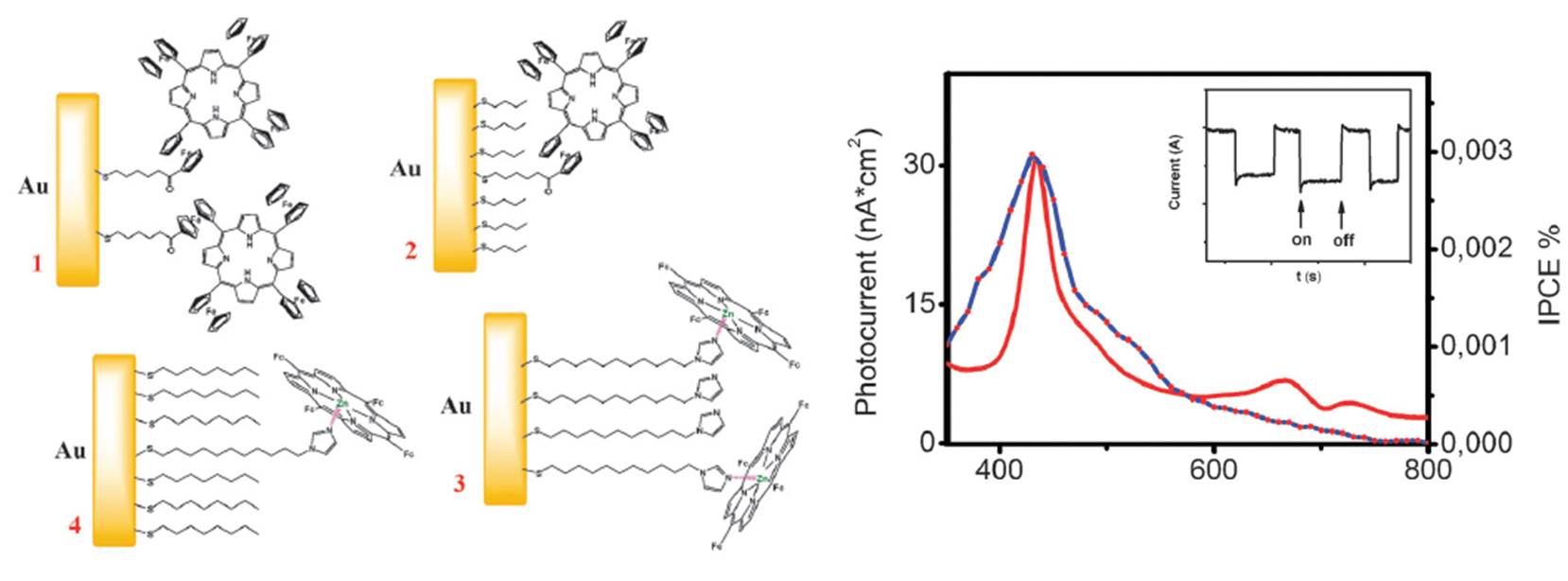
Representative publications: Vecchi, A.; Gatto, E.; Floris, B.; Conte, V.; Venanzi, M.; Nemykin, V. N.; Galloni, P. “Tetraferrocenylporphyrins as active components of self-assembled monolayers on gold surface”. Chem. Commun. , 2012 , 48, 5145-5147.
Axially substituted by ferrocene groups main-group porphyrins increase fluorescence upon oxidation and thus can be used as the redox-driven fluorescence markers or bio-markers:
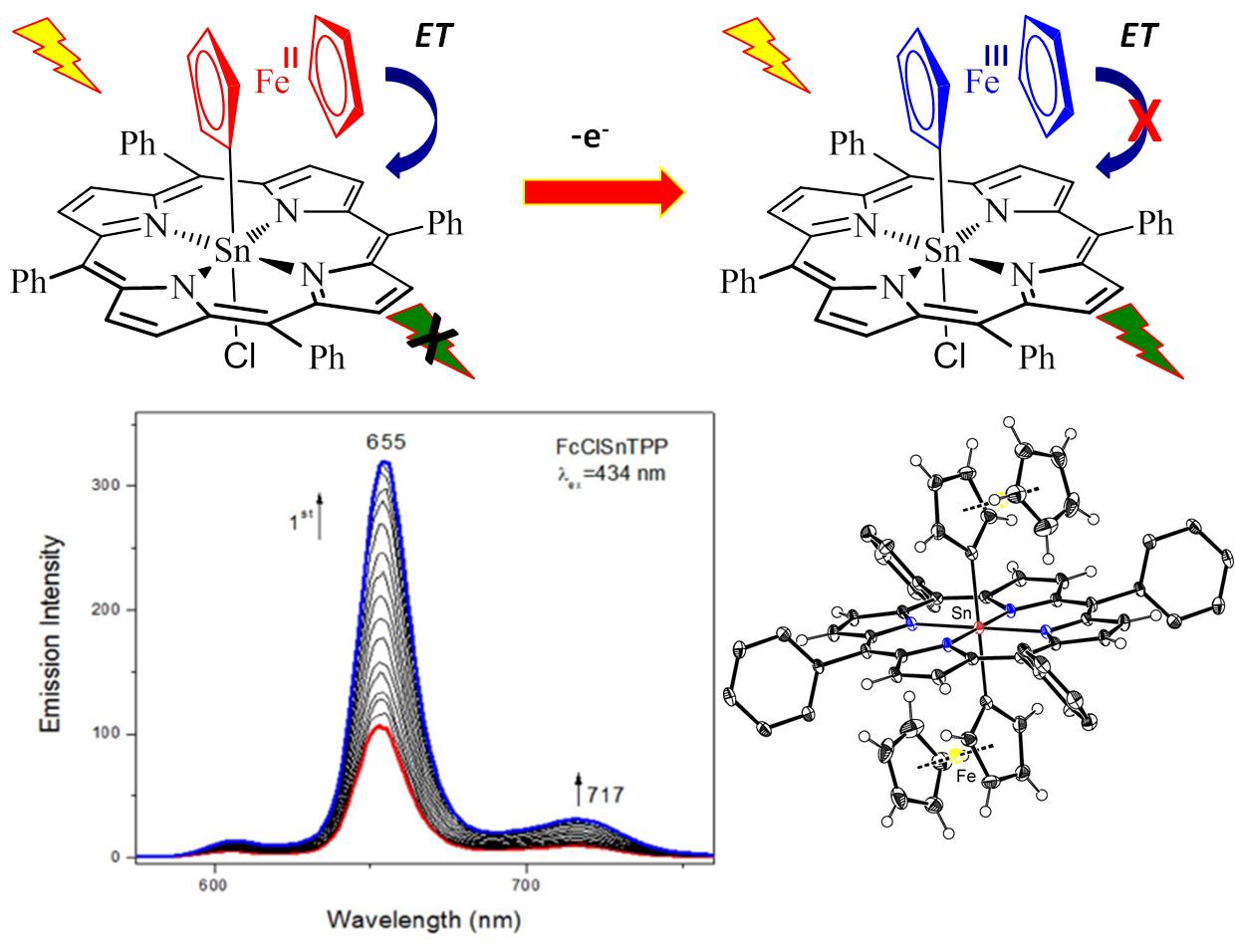
Representative publications: Solntsev, P. V.; Spurgin, K. L.; Sabin, J. R.; Heikal, A. A.; Nemykin, V. N. “Photoinduced Charge Transfer in Short-Distance Ferrocenylsubphthalocyanine Dyads”. Inorg. Chem. 2012 , 51 , 6537-6547. Solntsev, P. V.; Sabin, J. R.; Dammer, S. J.; Gerasimchuk, N. N.; Nemykin, V. N. “Unexpected fluorescence properties in the axially s -bonded ferrocenyl-containing porphyrin”. Chem. Commun. 2010 , 6581-6583.
We have shown that upon photoexcitation of the porphyrin core, ferrocene-containing porphyrins can efficiently transfer an electron from the organometallic substituent(s). These properties can be potentially used in Dye-Sensitized Solar Cells or Organic Photovoltaics. The key feature for the later application is the ability of organometallic porphyrins to co-crystallize with the efficient electron acceptors such as fullerenes. Examples of such “channeled” structures were recently discovered in our laboratory:
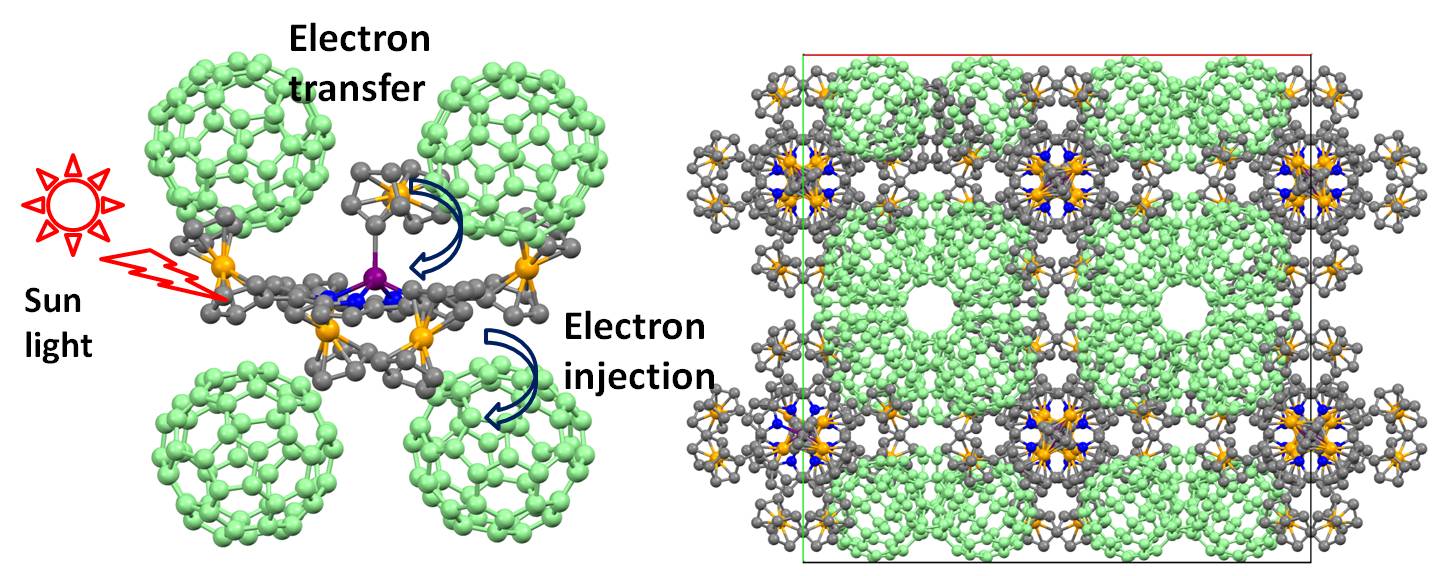
Representative publications: Solntsev, P. V.; Spurgin, K. L.; Sabin, J. R.; Heikal, A. A.; Nemykin, V. N. “Photoinduced Charge Transfer in Short-Distance Ferrocenylsubphthalocyanine Dyads”. Inorg. Chem. 2012 , 51 , 6537-6547. Vecchi, A.; Gatto, E.; Floris, B.; Conte, V.; Venanzi, M.; Nemykin, V. N.; Galloni, P. “Tetraferrocenylporphyrins as active components of self-assembled monolayers on gold surface”. Chem. Commun. , 2012 , 48, 5145-5147.
2. Catalysts for C-H bond activation
Transition-metal phthalocyanines and analogues are efficient catalysts for C-H bond activation in aromatic and aliphatic compounds. We are interested in application of such systems in selective oxidative transformations of the complex organic substrates. Our efforts are aimed at the development of Green oxidation catalists, which can be used with recyclable hyper-valent iodine compounds:
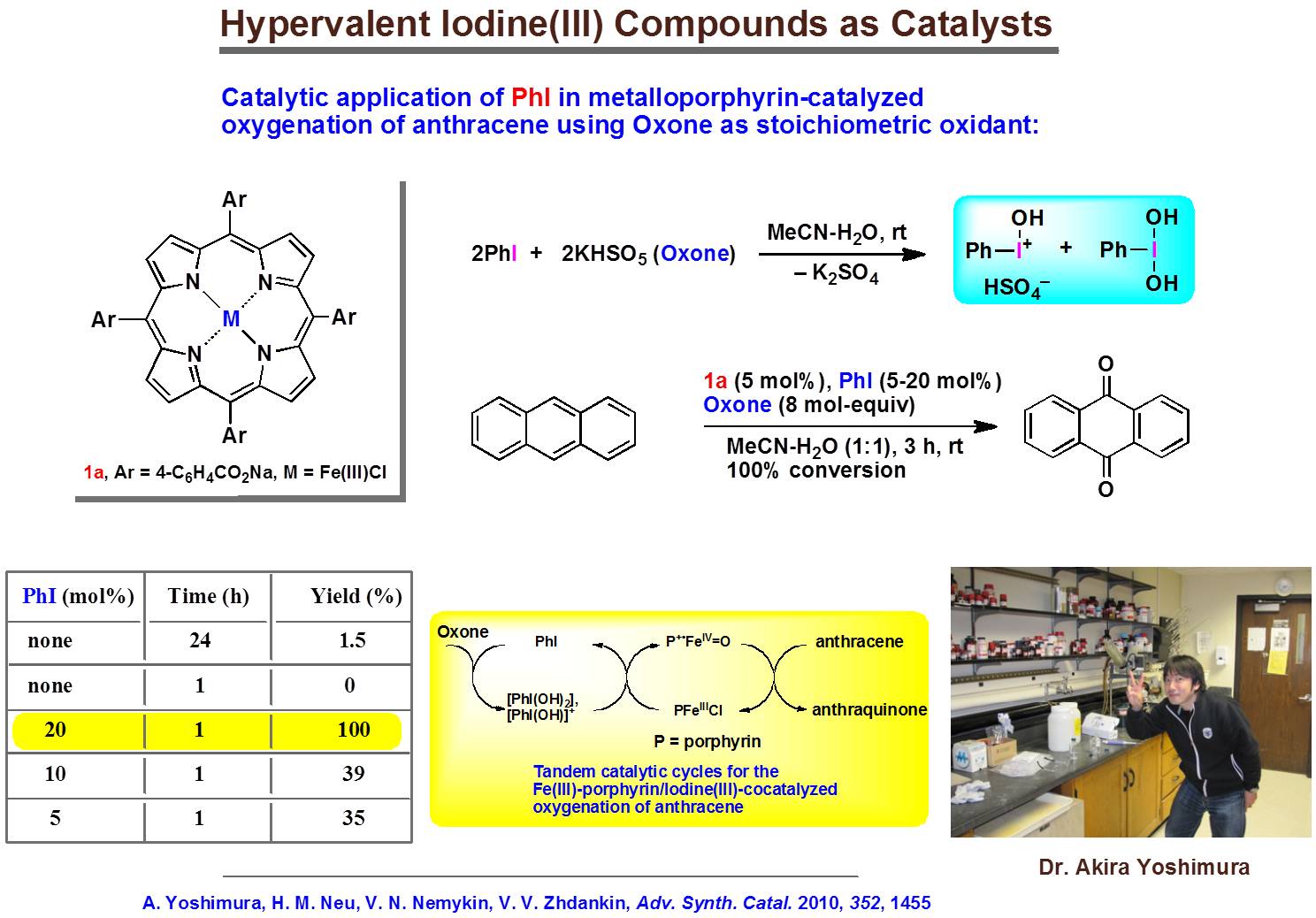
Picture is a courtesy of Professor Zhdankin (UMD).
Representative publications: Neu, H. M.; Zhdankin, V. V.; Nemykin, V. N. “Binuclear iron(III) phthalocyanine( m -oxodimer)/tetrabutylammonium oxone: a powerful catalytic system for oxidation of hydrocarbons in organic solution”. Tetr. Lett. 2010 , 51 , 6545-6548. Yusubov, M. S.; Nemykin, V. N.; Zhdankin, V. V. “Transition metal-mediated oxidations utilizing monomeric iodosyl- and iodylarene species”. Tetrahedron 2010 , 66 , 5745-5752. Yoshimura, A.; Neu, H. M.; Nemykin, V. N.; Zhdankin, V. V. “Metalloporphyrin/Iodine(III)-Cocatalyzed Oxygenation of Aromatic Hydrocarbons”. Adv. Synth. Catal. 2010 , 352 , 1455-1460. Neu, H. M.; Yusubov, M. S.; Zhdankin, V. V.; Nemykin, V. N. "Binuclear iron(III) phthalocyanine(µ-oxo-dimer)-catalyzed oxygenation of aromatic hydrocarbons with iodosylbenzene sulfate and iodosylbenzene as the oxidants". Adv. Synth. Catal. 2009 , 351 , 3168-3174. Geraskin, I. M.; Pavlova, O.; Neu, H. M.; Yusubov, M. S.; Nemykin, V. N.; Zhdankin, V. V. " Comparative reactivity of hypervalent iodine oxidants in metalloporphyrin-catalyzed oxygenation of hydrocarbons: iodosylbenzene sulfate and 2-iodylbenzoic acid ester as safe and convenient alternatives to iodosylbenzene". Adv. Synth. Catal. 2009 , 351 , 733-737. Geraskin, I. M.; Luedtke, M. W.; Neu, H. M.; Nemykin, V. N.; Zhdankin, V. V. " Organic iodine(V) compounds as terminal oxidants in iron(III) phthalocyanine catalyzed oxidation of alcohols". Tetr. Lett. 2008 , 49 , 7410-7412 .
3. Magnetic Circular Dichroism (MCD) spectroscopy and electronic structure of porphyrinoids
MCD spectroscopy an additional insight into the electronic structure and excited state properties of porphyrins and analogues provides. It compliments electronic absorption spectroscopy and allows accurate assignments of the excited states in target compounds. Energies and origins of such excited states could further be confirmed and refined by the Density Functional Theory (DFT) and time-dependent DFT (TDDFT) calculations, which are routine methods in our laboratory. We also are interested in development of computational approaches and use of modern DFT, CAS-SCF, and MRCI methods for the calculation and accurate prediction of the absorption, NMR, IR, EPR, and Mössbauer spectral parameters in the transition-metal complexes (VMOdes page is here and some useful tips on Gaussian program are here).
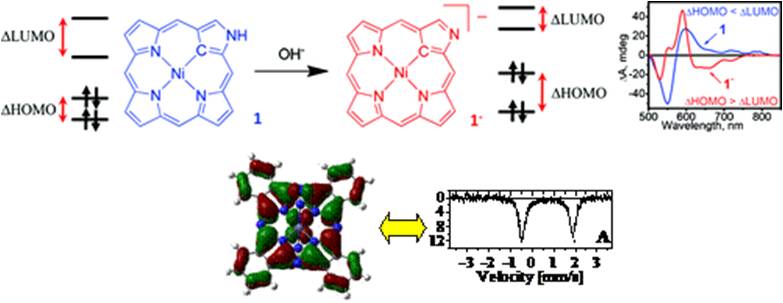
Representative publications: Sabin, J. R.; Varzatskii, O. A.; Voloshin, Y. Z.; Starikova, Z. A.; Novikov, V. V.; Nemykin, V. N. “Insight into the Electronic Structure, Optical Properties, And Redox Behavior of the Hybrid Phthalocyaninoclathrochelates from Experimental and Density Functional Theory Approaches”. Inorg. Chem. , 2012 , 51 , 8362-8372. Nemykin, V. N.; Sabin, J. R. “Profiling Energetics and Spectroscopic Signatures in Prototropic Tautomers of Asymmetric Phthalocyanine Analogues”. J. Phys. Chem. A 2012 , 116 , 7364-7371. Ziegler, C. J.; Sabin, J. R.; Geier, G. R.; Nemykin, V. N. “The first TDDFT and MCD studies of free base triarylcorroles: A closer look into solvent-dependent UV-visible absorption”. Chem. Commun. , 2012 , 48 , 4743-4745. Nemykin, V. N.; Polshyna, A. E.; Makarova, E. A.; Kobayashi, N.; Lukyanets, E. A. “Unexpected formation of the nickel seco-tribenzoporphyrazine with a tribenzotetraazachlorin-type absorption spectrum”. Chem. Commun. , 2012 , 48 , 3650–3652. Sripothongnak, S.; Ziegler, C. J.; Dahlby, M. R.; Nemykin, V. N. “Controllable and Reversible Inversion of the Electronic Structure in Nickel N-Confused Porphyrin: A Case When MCD Matters”. Inorg. Chem. , 2011 , 50 , 6902-6909 . Nemykin, V. N.; Hadt, R. G. “ Interpretation of the UV-Vis spectra of the meso(ferrocenyl)-containing porphyrins using TDDFT approach: is Gouterman's classic four-orbital model still in play?”. J. Phys. Chem. A 2010 , 114 , 12062-12066. Nemykin, V. N.; Hadt, R. G.; Belosludov, R. V.; Mizuseki, H.; Kawazoe, Y. "Influence of Molecular Geometry, Exchange-Correlation Functional, and Solvent Effects in the Modeling of Vertical Excitation Energies in Phthalocyanines Using TDDFT and PCM-TDDFT Methods: Can Modern Computational Chemistry Methods Explain Experimental Controversies?" J. Phys. Chem. A 2007 , 111 , 12901-12913.
4. Secondary interactions in the hyper-valent iodine compounds
In collaboration with Professor Viktor Zhdankin, we are interested in formation of the supramolecular assemblies and macrocycles based on iodine(III) and iodine(V) compounds. Triple-helix and macrocyclic structures of two iodine(III) compounds are presented below:
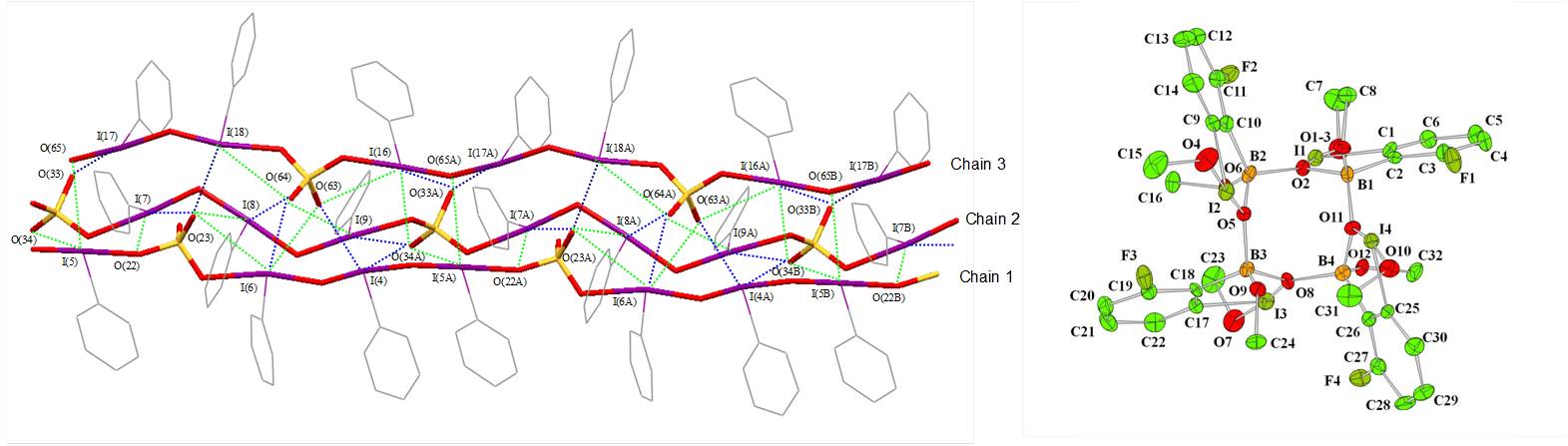
Representative publications: Yusubov, M. S.; Yusubova, R. Y.; Nemykin, V. N.; Maskaev, A. V.; Geraskina, M. R.; Kirschning, A.; Zhdankin, V. V. “Potassium 4-iodylbenzenesulfonate: preparation, structure, and application as a reagent for oxidative iodination of arenes”. Eur. J. Org. Chem. 2012 , in press , DOI: 10.1002/ejoc.201201064. Nemykin, V. N.; Maskaev, A. V.; Geraskina, M. R.; Yusubov, M. S.; Zhdankin, V. V. “Preparation and X-ray crystal study of benziodoxaborole derivatives: new hypervalent iodine heterocycles”. Inorg. Chem. , 2011 , 50 , 11263-11272. Nemykin, V. N.; Koposov, A. Y.; Netzel, B. C.; Yusubov, M. S.; Zhdankin, V. V. " Self-Assembly of Hydroxy(phenyl)iodonium Ions in Acidic Aqueous Solution: Preparation, and X-ray Crystal Structures of Oligomeric Phenyliodine(III) Sulfates". Inorg. Chem. 2009 , 48 , 4908-4917.
5. Experimental and theoretical modeling of the oxygen-atom transfer reactivity in molybdenum complexes
Our main main focus in this project is: i) modeling of oxygen-atom transfer reactivity and electron pathway in the mononuclear tungstopterin enzymes; ii) compounds for molecular electronics and mixed-valence complexes; iii) prediction of the electronic structure, stability, and spectroscopic properties of transition-metal complexes. Recently, we have reported the several new molybdenum complexes, which exhibit discrete oxygen atom transfer reactivity and were able to isolate key reaction intermediates. Our current bio-inorganic projects are dealing with the different “scorpionate” and dithiolate tungsten complexes as well as with iron-sulfur clusters:
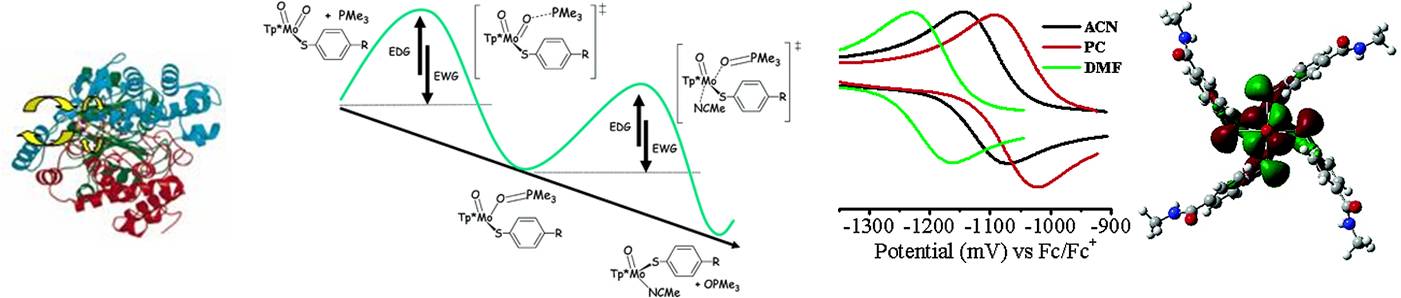
Representative publications: Hadt, R. G.; Nemykin, V. N.; Olsen, J. G.; Basu, P. "Comparative calculation of EPR spectral parameters in [Mo V OX 4 ] - , [Mo V OX 5 ] 2- , and [Mo V OX 4 (H 2 O)] - complexes". Phys. Chem. Chem. Phys. 2009 , 11 , 10377-10384. Basu, P.; Nemykin, V. N.; Sengar, R. S. " Substituent Effect on Oxygen Atom Transfer Reactivity from Oxomolybdenum Centers: Synthesis, Structure, Electrochemistry, and Mechanism". Inorg. Chem. 2009 , 48 , 6303-6313. Sengar, R. S.; Nemykin, V. N., Basu, P. "Synthesis, Electrochemistry, Geometric and Electronic Structure of Oxo-Molybdenum Compounds Involved in an Oxygen Atom Transferring System". J. Inorg. Biochem. 2008 , 102 , 748-756.
Prof. Viktor Zhdankin, UMD; Prof. Paul Kiprof, UMD; Prof. Steven Berry, UMD; Prof. James Riehl, UMD; Prof. David Blank, UMN; Prof. Barbara Floris and Prof. Pierluca Galloni, University of Rome; Prof. Partha Basu, Duquesne University; Prof. Edward Solomon, Stanford University; Prof. Chris Ziegler, University of Akron; Prof. Karl Kadish, University of Houston; Prof. Alex Nazarenko, Buffalo State College; Prof. Nick Gerasimchuk, Southern Missouri State University; Dr. Douglas Fox, Gaussian Inc.; Prof. Keichii Sakamoto, Nihon University, Japan; Prof. Rodion Belosludov, Tohoku University, Japan; Prof. Eugenii A. Lukyanets, NIOPIK, Russia; Prof. Yuriy P. Kovtun, NASU, Ukraine
(and many other groups!)
© 2017 Victor Nemykin, All Rights Reserved
Last Updated October 02, 2017