(G. Schreckenbach, T. Ziegler, J. Phys. Chem. A 1997, 101, 3388-3399)

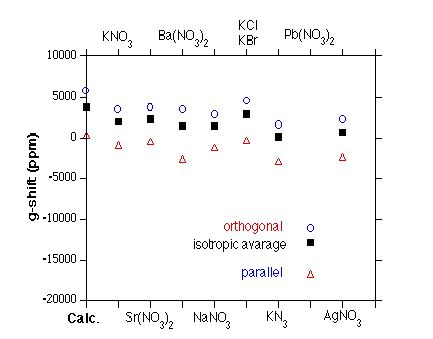
Figure 1.
Experimental and calculated g-shifts of the NO32- radical in different host matrices. The g-shift (tensor) is defined as the deviation of the molecular g-value (g-tensor)from the free electron g-value of 2.0023192778. The figure illustrates that the experimental range
for the g-shift is enormous, making the comparison with the calculated results (which
are based on a single, rigid molecule, i.e., on the zero-pressure, zero-temperature
limit of a gas phase experiment) very difficult if not impossible. Note,
however, that the calculated numbers exhibit the right trend with the orthogonal g-shift tensor components being much larger then the component parallel to
the threefold symmetry axis. The calculated anisotroy, i.e., the difference
between parallel and orthognal principal tensor components, has the correct
sign and right order of magnitude.
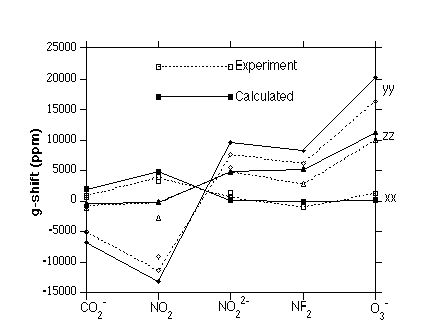
Figure 2.
Principal tensor components of the g-shift in symmetric AB2 radicals of first-row compounds. The g-shift is defined in the caption to Figure 1. The axis system was chosen
such that the molecules lie in the yz-plane, and that the z-axis coincides with the twofold symmetry axis. Experimental trends, both
regarding the principal tensor components and trends between related molecules,
are well reproduced.
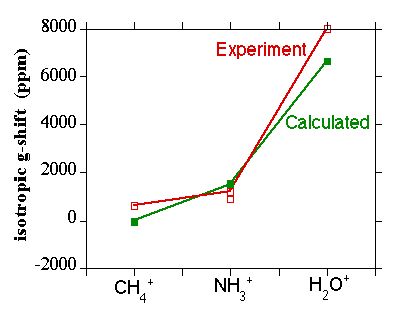
Figure 3.
Isotropic g-shift in CH4+, NH3+, and H2O+.
![]() Back to Georg's Home Page
Back to Georg's Home Page
Back to Georg's Publications
Back to Research Page
© Georg Schreckenbach, 1997-2001