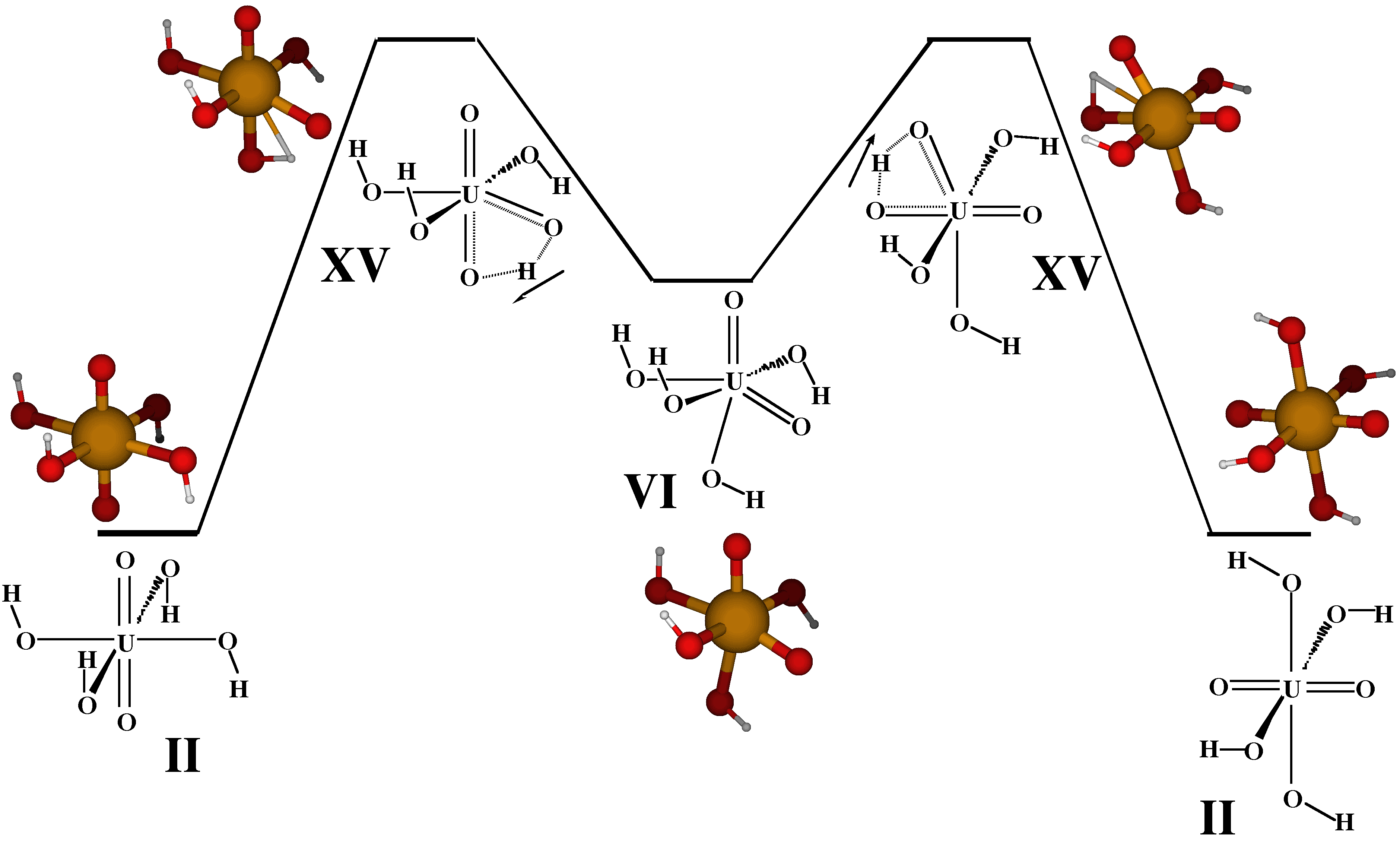

Figure.
Proposed non-aqueous potential energy surface for the intramolecular exchange
of uranyl and hydroxide oxygen atoms in uranyl tetrahydroxide (VI), UO2(OH)42-. The transition state, XV, and the stable intermediate "cis-uranyl" structure, VI, are calculated to have total energies relative to II of 37.5 kcal/mol and 19.1 kcal/mol, respectively. Calculations are based
on density functional theory (DFT) with the so-called B3LYP hybrid exchange-correlation
functional and a newly developed relativistic effective core potential on
the actinide center. (G. Schreckenbach, P. Jeffrey Hay, Richard L. Martin, Inorg. Chem. 1998, 37, 4442 )
![]() Back to Georg's Home Page
Back to Georg's Home Page
Back to Georg's Publications
Back to Research Page
© Georg Schreckenbach, 1997-2001