|
Environmental Chemistry and Biogeochemistry Group |
|||
|
Home People Research Opportunities Courses Publications News UCTEL SERF CEOS CHRFEER
|
|||
|
Research Interests:
We study environmental chemistry and biogeochemistry of contaminants, especially in the Arctic marine environment. Such studies are carried out at both the molecular scale to probe the fundamental chemical mechanisms, and the regional to global scales to address the interplay between chemical contamination and climate change.
Major research activities in our group fall in three areas: 1) Biogeochemistry of mercury and other trace elements; 2) Cryo-reactions and Arctic marine cryospheric chemistry; and 3) Interplay between chemical contamination and climate change.
1. Biogeochemistry of Mercury and Other Trace Elements
Many contaminants are studied in our laboratory, though the main focus has been on mercury, because it is not only a legacy contaminant of primary concern in the Arctic marine ecosystem, but also a promising "tracer" for changing biogeochemical processes in the environment.
Our research aims to fill critical knowledge gaps in chemical and biogeochemical processes governing mercury transport and effects across environmental and bio-interfaces, and how these processes respond to a changing climate. Major processes studied include:
1) Role of sulfides, polysulfides, thiols, and selenides in mercury speciation and toxicity. We developed the first and only analytical method for the speciation of methylmercury-thiol complexes in biological samples (Lemes and Wang, 2009). We were the first to report direct analytical evidence of the presence and dominance of methylmercuric cysteinate, a complex that is at the center of mercury neurotoxicity, in fish muscle (Lemes and Wang, 2009), beluga tissues (Lemes et al., 2011), and rice grains (Li et al., 2010). We discovered a new pathway for methylmercury demethylation involving selenoamino acids (Khan and Wang, 2010), and two new pathways for biomineralization of Hg-Se-S nanoparticles (Khan and Wang, 2009, 2010).
2) Transport and transformation of mercury in the Arctic Ocean and Himalayas. We were the first to study mercury transport and transformation in the Arctic sea ice environment (Chaulk et al., 2011; Burt et al., 2013; Beattie et al., 2014; Xu et al., 2016), and reported the first evidence supporting in situ mercury methylation in multi-year ice (Beattie et al., 2014) and in sub-surface Arctic seawater (Wang et al., 2012). We show that the sub-surface methylmercury enrichment in seawater can readily explain why animals in the western Canadian Arctic have higher mercury than their counterparts in the eastern region (Wang et al., 2018). We have also shown that mercury can be transported to the remote Tibetan Plateau and Himalayas via dust storms (Loewen et al., 2007; Zhang et al., 2012), and that the mercury record in glaciers can be used to establish the chronology of not only mercury emissions (Kang et al., 2016) but also ice accumulation (Kang et al., 2015).
3) Mercury chemistry in the atmosphere. By comparing the Equatorial Pacific with the Arctic, we discovered a missing pathway in mercury oxidation in the marine boundary layer (Wang et al., 2014). We were also part of a team that discovered a major role of gaseous phase photo-reduction of mercury in the global atmosphere (Saiz-Lopez et al., 2018).
2. Cryo-reactions and Arctic Marine Cryospheric Chemistry
Most chemical reactions in aqueous solution slow down as temperature decreases. In frozen media, however, certain reactions are known to proceed very differently from their aqueous counterparts, some being accelerated in rate while others yielding unexpected products. These sub-zero temperature (Celsius) chemical processes, or "cryo-reactions", are increasingly recognized as being environmentally important, playing a critical role in not just atmospheric processes, but also in the surface cryosphere. We discovered that pH in sea ice can differ over two orders of magnitude (Hare et al., 2013). We have extended the solubility function of CO2 to seawater and sea ice brine at sub-zero temperatures (Bailey et al., 2018), and made major progress toward the fundamental understanding of a new CO2 exchange mechanism between the atmosphere and the ocean via the formation and melting of ikaite in sea ice ("sea ice pump") (Rysgaard et al., 2013, 2014). We also discovered the formation of cryogenic gypsum in sea ice (Geilfus et al., 2013). Our studies on mercury and other contaminants in sea ice have resulted in an invited chapter on contaminants transport and transformation in the sea ice environment (Wang et al., 2017) in the new edition of Sea Ice (Thomas, 2017).
With the recent development of the Churchill Marine Observatory, we are increasingly involved in the study of oil and transportation-related contaminants in sea ice-covered waters, as they are contaminants of emerging concern in the Arctic due to the projected increase in industrial activities associated with marine shipping and oil and gas extraction.
3. Interplay between chemical contamination and climate change
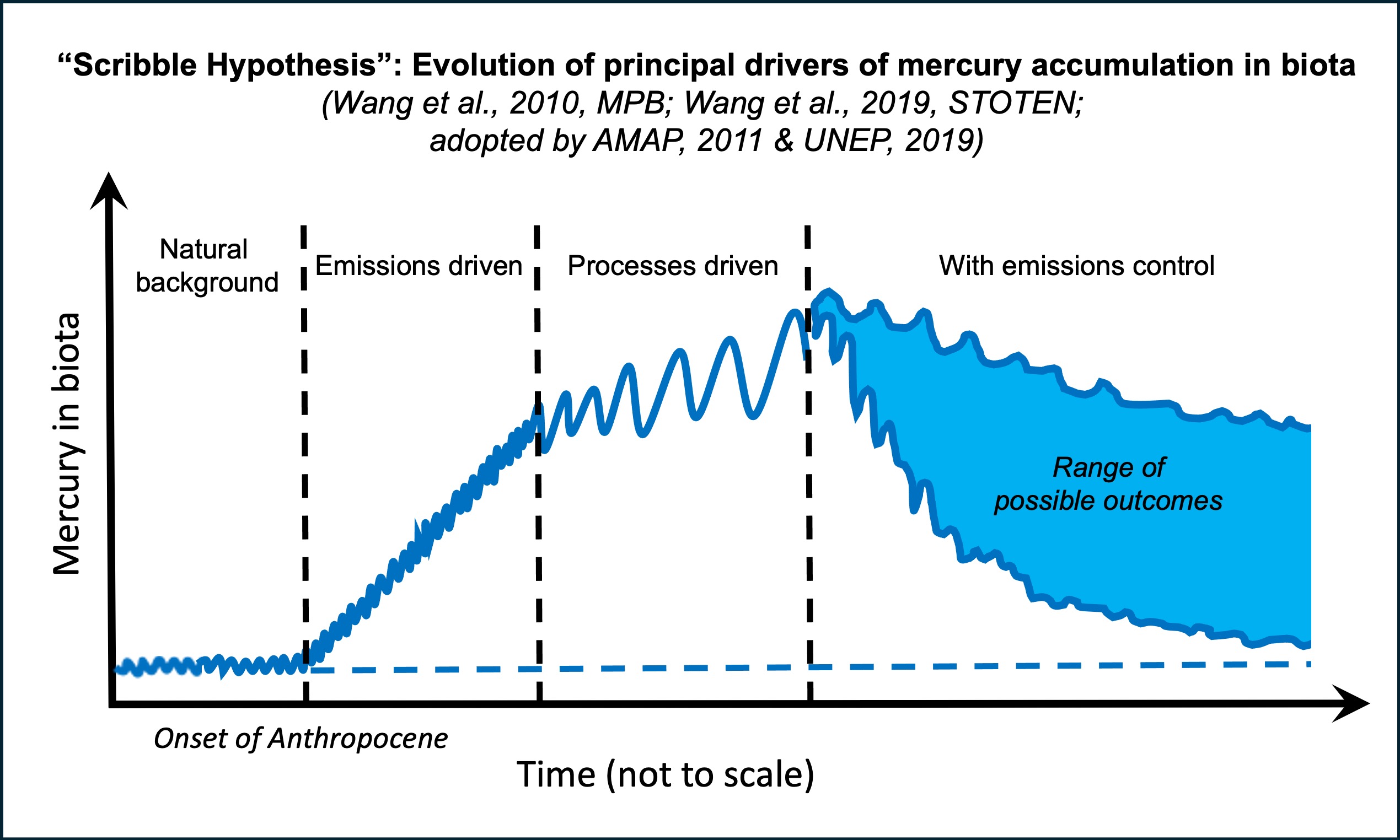
Among the most significant impact our research has made is our postulation that during rapid climate change, emission control of contaminants may be followed by long delays before ensuing reduction is seen in food-web contaminant levels (Wang et al., 2010; Wang et al., 2019) (figure above). As the contaminant flux to many environmental reservoirs has increased dramatically since the Anthropocene, we proposed that bioaccumulation of contaminants would be increasingly driven by climate-induced changes in internal biogeochemical processes and ecological dynamics rather than by additional input from the external sources. We further proposed that this paradigm change from "source-driven" to "process-driven" in contaminant bioaccumulation would be most profound in the Arctic where climate change occurs rapidly. This postulation has since been validated by recent studies from my group and by the larger scientific community. Our postulation and its major policy implications are highlighted in the AMAP (Arctic Monitoring and Assessment Programme) reports of the Arctic Council (AMAP, 2011a,b) and the UNEP (United Nations Environment Programme) Global Mercury Assessment - 2018.
|
|
Research Facilities:
Major research facilities supporting our research include: Ultra-Clean Trace Elements Laboratory (UCTEL) Petroleum Environmental Research Laboratory (PETRL) Sea-ice Environmental Research Facility (SERF) Churchill Marine Observatory (CMO) Canadian Research Icebreaker CCGS Amundsen Manitoba Institute for Materials
Laboratory-Scale
Ultra-Clean Trace Elements Laboratory (UCTEL)
Mesocosm-Scale
Sea-ice Environmental Research Facility (SERF)
Field-Scale
|
|
|
|
|||
|
|
|||