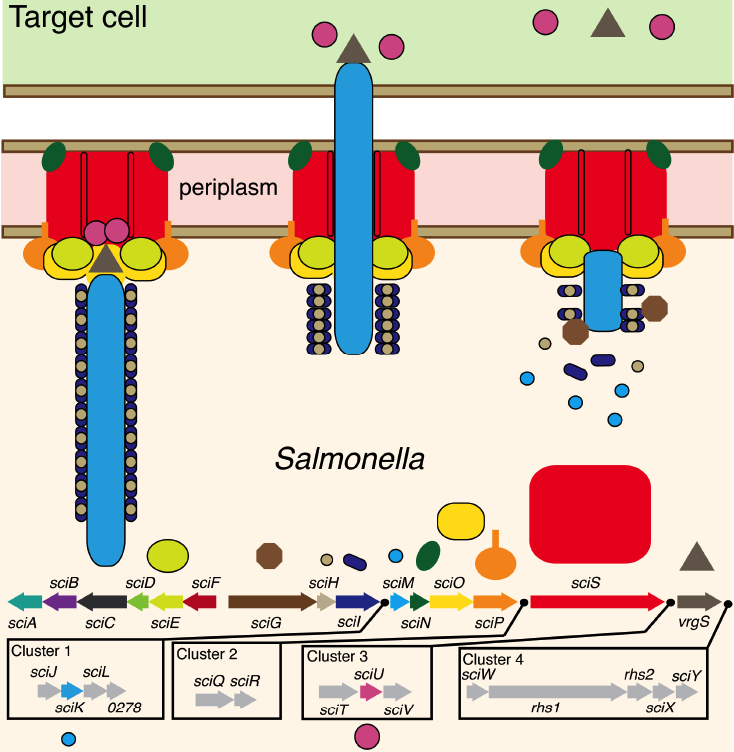
Cartoon diagram of the
Salmonella T6SS.The type VI secretion system
Type VI secretion systems (T6SS) are versatile and dynamic nanomachines that Gram-negative bacteria have adapted for numerous different biological functions. These include strain competition and killing of non-self bacteria, self-recognition, natural competence, direct communication with a mammalian host by the microbiotia, and pathogenesis. Specifically, the T6SS has been shown to provide direct communication at the molecular level with mammalian, avian, reptile, and plant hosts
The T6SS is used to forcibly inject effectors into both prokaryotic and eukaryotic cells. Although the apparatus is conserved, many species have numerous uncharacterized extra open reading frames. These non-core genes and/or auxiliary gene clusters are hypothesized to be regulators and effectors of the system.
Type VI secretion systems (T6SS) are versatile and dynamic nanomachines that Gram-negative bacteria have adapted for numerous different biological functions. These include strain competition and killing of non-self bacteria, self-recognition, natural competence, direct communication with a mammalian host by the microbiotia, and pathogenesis. Specifically, the T6SS has been shown to provide direct communication at the molecular level with mammalian, avian, reptile, and plant hosts
The T6SS is used to forcibly inject effectors into both prokaryotic and eukaryotic cells. Although the apparatus is conserved, many species have numerous uncharacterized extra open reading frames. These non-core genes and/or auxiliary gene clusters are hypothesized to be regulators and effectors of the system.
Versatility and Adaptation of the T6SS
The Salmonella T6SS
Salmonella as a species has five T6SSs, suggesting that different T6SS may be adapted for varied purposes and different hosts. For example, the T6SS present in SPI-6 (Salmonella pathogenicity island) is required for mammalian infection by Salmonella Typhimurium, the SPI-19 T6SS contributes to colonization in chickens for S. gallinarium, and S. bongori that infect reptiles possess SPI-22, that harbors a phylogenetically distinct T6SS.
My lab is interested in understanding how the T6SS is adapted at the molecular level for its various functions and hosts using Salmonella as a model system.
For this work we collaborate with the laboratory of Ethel Bayor-Santos to characterize the diversity of T6SS Salmonella effectors.
Salmonella as a species has five T6SSs, suggesting that different T6SS may be adapted for varied purposes and different hosts. For example, the T6SS present in SPI-6 (Salmonella pathogenicity island) is required for mammalian infection by Salmonella Typhimurium, the SPI-19 T6SS contributes to colonization in chickens for S. gallinarium, and S. bongori that infect reptiles possess SPI-22, that harbors a phylogenetically distinct T6SS.
My lab is interested in understanding how the T6SS is adapted at the molecular level for its various functions and hosts using Salmonella as a model system.
For this work we collaborate with the laboratory of Ethel Bayor-Santos to characterize the diversity of T6SS Salmonella effectors.
Protein Secretion Mechanisms of the T6SS
Membrane protein effectors
Gram-negative bacteria have multiple mechanisms to load effector toxins onto the T6SS for delivery into prey-cells. In one mechanism, the effector toxins contain a motif termed pre-PAAR and a domain termed PAAR that help load the toxin onto the tip of the T6SS. Additionally, these effectors also contain transmembrane domains that are thought to catalyze membrane penetration and localization to the cytoplasm of a prey-cell.
Our lab is interested in characterizing the molecular mechanism of membrane protein effector loading onto the T6SS, and the biophysical process of how the effector transmembrane domains catalyze prey-cell membrane transport into the cytoplasm.
We collaborate with the laboratory of John Whitney to study membrane protein effector secretion.
Gram-negative bacteria have multiple mechanisms to load effector toxins onto the T6SS for delivery into prey-cells. In one mechanism, the effector toxins contain a motif termed pre-PAAR and a domain termed PAAR that help load the toxin onto the tip of the T6SS. Additionally, these effectors also contain transmembrane domains that are thought to catalyze membrane penetration and localization to the cytoplasm of a prey-cell.
Our lab is interested in characterizing the molecular mechanism of membrane protein effector loading onto the T6SS, and the biophysical process of how the effector transmembrane domains catalyze prey-cell membrane transport into the cytoplasm.
We collaborate with the laboratory of John Whitney to study membrane protein effector secretion.
