
ORIGINS OF CANCER
The loss of Chromosome Stability is referred to as Chromosome Instability or CIN. CIN is a hallmark of cancer, marked by an increased rate of chromosome gains or losses within cells. This genomic chaos drives genetic diversity, fueling early disease development, tumor progression, metastasis, and resistance to therapy, all of which contribute to poor patient outcomes. CIN is highly prevalent in many cancers, including colorectal and ovarian cancers, where it underlies the aggressive nature and adaptability of tumors.
Despite its critical role, the aberrant genes driving CIN, known as CIN genes, remain largely unknown. By leveraging innovative single-cell and quantitative imaging microscopy (QuantIM) technologies, our research is at the forefront of discovering these novel CIN genes. Identifying cancer-specific CIN genes not only deepens our understanding of how cancers arise and evolve but also creates new opportunities to develop innovative Precision Medicine strategies. Targeting these unique vulnerabilities enables the development of highly selective therapies, offering hope for more effective and personalized cancer treatments.

We invite passionate trainees to join us in this groundbreaking work, where you’ll gain hands-on experience with cutting-edge tools and contribute to discoveries that reshape our understanding of cancer.
Our team is committed to uncovering the molecular determinants (i.e., aberrant genes, proteins, and pathways) that drive CIN, especially in colorectal and ovarian cancers. By leveraging cutting-edge single-cell and quantitative imaging microscopy (QuantIM) technologies, we aim to reveal the fundamental mechanisms behind CIN. This knowledge is not only crucial to better understand the aberrant biology driving disease development and progression, but is instrumental to develop innovative, precision medicine strategies that target the unique vulnerabilities created by CIN, offering new hope for more effective and personalized cancer therapies.
Hallmarks of Cancer: Focus on Emerging and Original Traits
Cancer cells acquire a set of defining characteristics known as the hallmarks of cancer, which enable their uncontrolled growth and survival. Our research concentrates on three critical hallmarks: the emerging hallmark of genome instability and mutation, alongside the original hallmarks of evading growth suppressors and sustaining proliferative signaling. These interconnected processes are fundamental drivers of tumor initiation and progression, particularly in colorectal and ovarian cancers, where they create the conditions for malignant transformation and aggressive disease behavior.
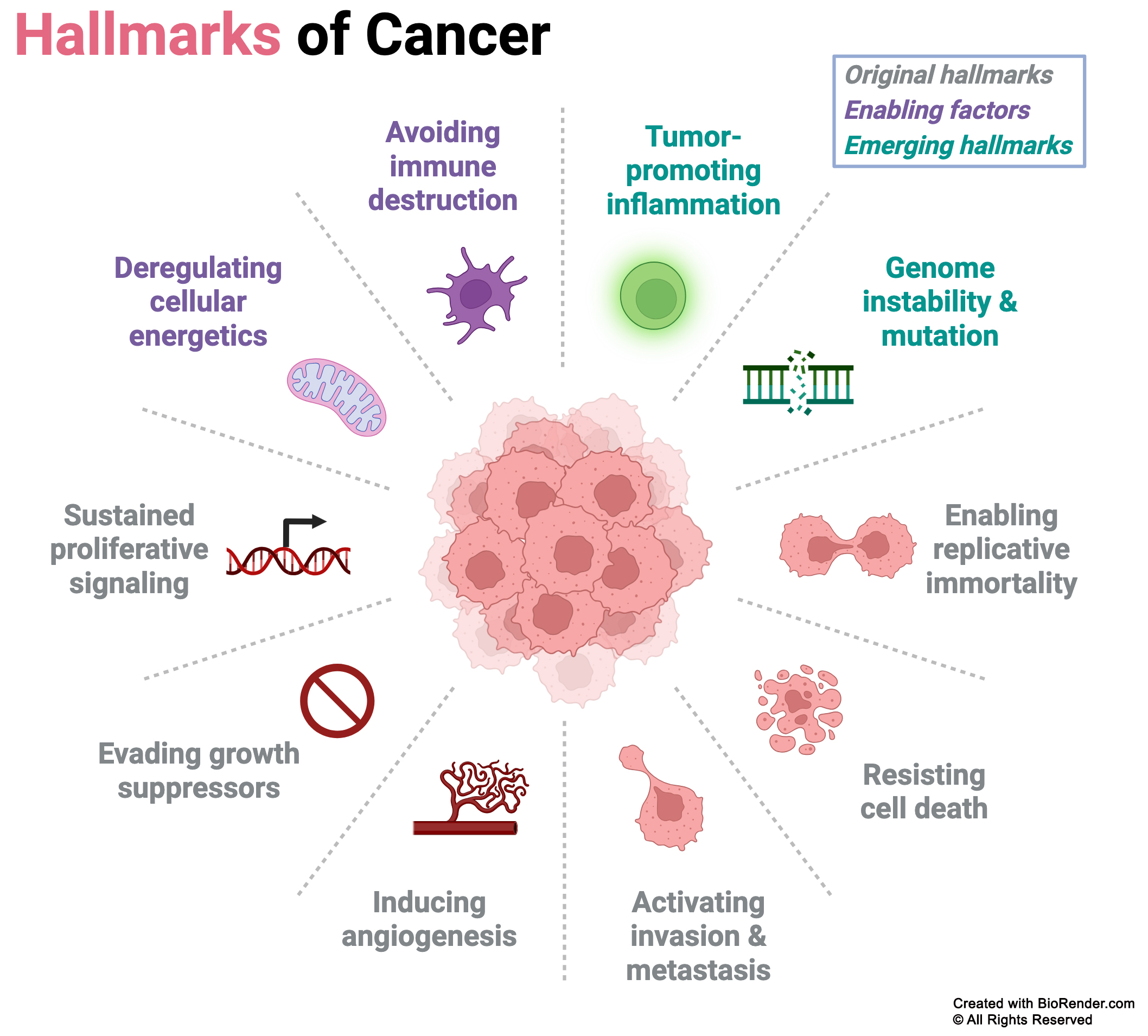
The classic hallmarks of cancer, highlighting the key biological traits that enable tumor cells to thrive and spread. Our research is particularly focused on three interconnected hallmarks: genome instability and mutation, evading growth suppressors, and sustained proliferative signaling.
CIN as a Central Hub in Cancer Development and Patient Outcomes
CIN represents a central hub that fuels multiple stages of cancer, from early cellular transformation and tumor evolution to metastasis (disease spread), treatment resistance, and ultimately poor patient outcomes. CIN generates genetic diversity within tumors, promoting clonal selection and enabling cancer cells to adapt and survive therapeutic pressures. This dynamic genomic chaos underlies the aggressive nature of many cancers and is a key factor in their ability to evade many current treatments.
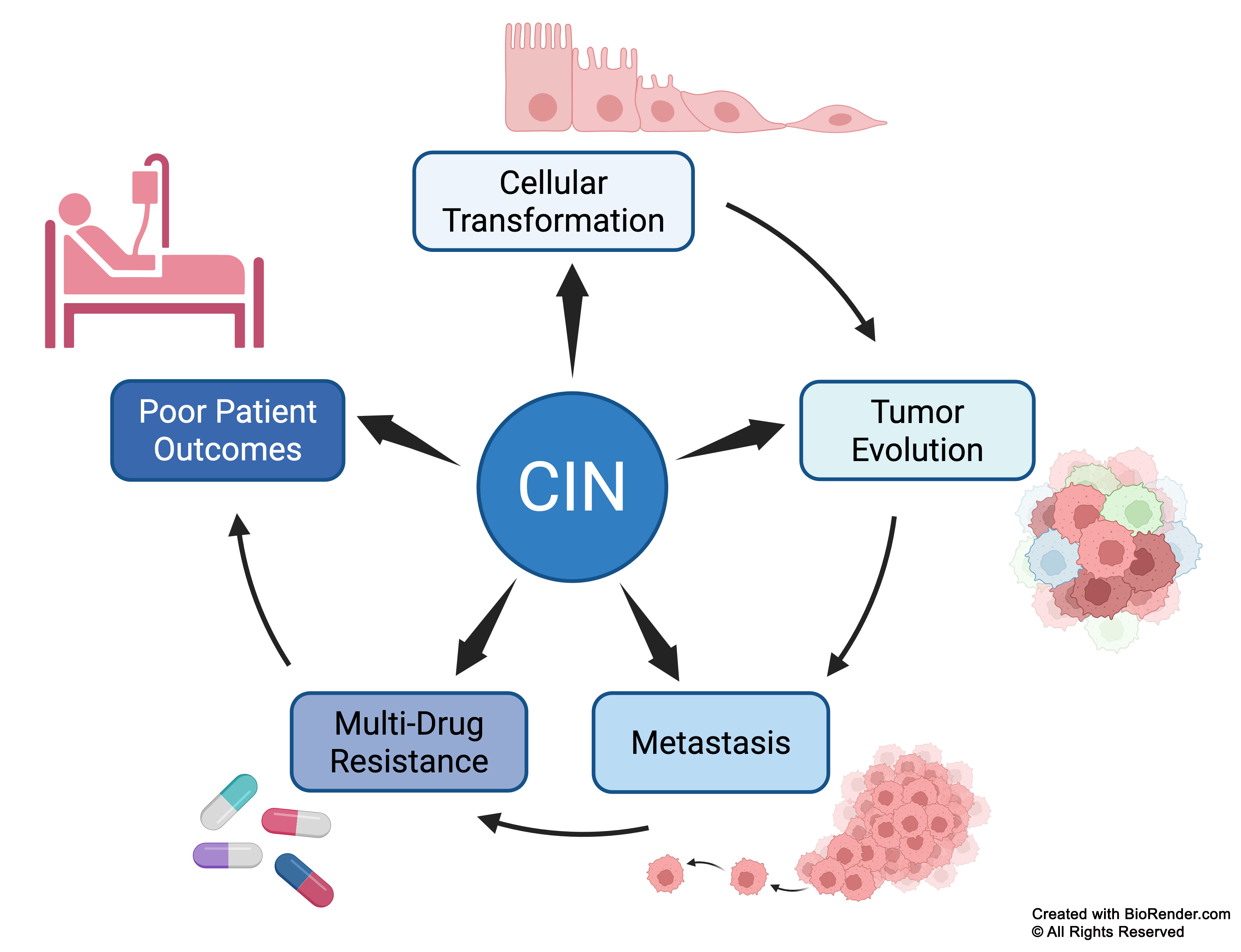
Schematic highlighting CIN as the central driver of cancer progression. CIN fuels cellular transformation, tumor evolution, metastasis, multi-drug resistance, and poor patient outcomes by promoting clonal selection and the emergence of resistant cancer cells during treatment.
By focusing on how CIN underpins key cancer hallmarks, we are uncovering the molecular origins of cancer. Our innovative research aims to reveal new vulnerabilities in cancer cells, opening the door to precision medicine approaches that target the root causes of tumor growth and resistance.