RESEARCH FOCUS
Our lab investigates how our cells protect their genetic information and what happens when these protective systems fail. We focus on the genes, proteins, and biological pathways that maintaining genome integrity and chromosome stability that are essential for preventing diseases like cancer. When these safeguards break down, errors can accumulate in our DNA, leading to cancer development, progression, and influencing how tumors respond to treatment.
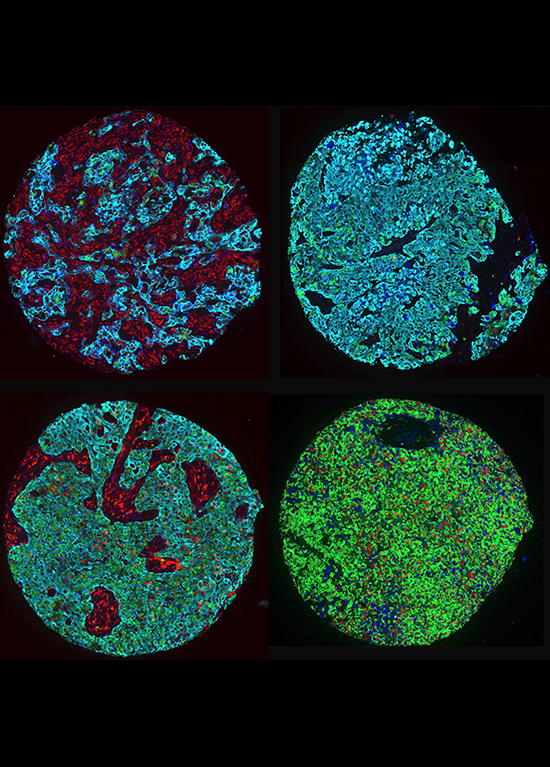
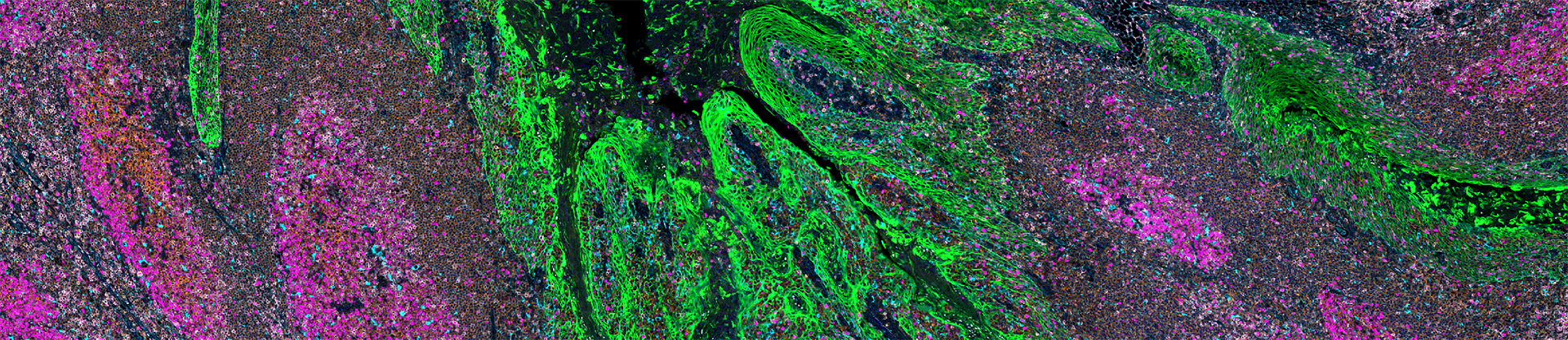
By studying these processes, we aim to uncover specific weaknesses in cancer cells that can be targeted with new, more precise therapies, reducing harm to healthy cells to limit adverse side effects.
We are focused on addressing three key questions:
1. Which genes, proteins, and pathways safeguard our genome and chromosome stability under normal conditions?
2. How do disruptions in these molecular systems drive chromosome instability to influence cancer development, progression, and treatment response?
3. Can we identify new drug targets that selectively target cancer cells with chromosome instability, while leaving healthy cells unharmed?
Through this work, we aim to deepen our fundamental understanding of genome/chromosome biology and accelerate the discovery of innovative cancer therapies.
FUNDING PARTNERS
We sincerely thank the CancerCare Manitoba Foundation and the generous donors throughout Manitoba for their vital support of our trainees, operations, and infrastructure.
We also gratefully acknowledge the following organizations for funding our trainees and research, for without these contributions, our research would not be possible.
Canadian Institutes of Health Research (CIHR)
CancerCare Manitoba (CCMF)
Natural Sciences & Engineering Research Council of Canada (NSERC)
Research Manitoba (RM)