The Molecules of Life: Biochemistry
Carbohydrates
(Canadian Campbell 2nd ed,- Concept 5.2)
Carbohydrates serve as energy sources and provide structural support as
in the cell wall of plants. Carbon, hydrogen, and oxygen are the elements found
in carbohydrates.
Exercise 1 - Benedict's Test for Reducing
Sugars
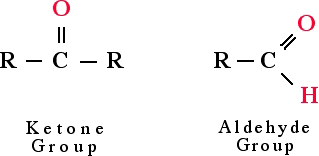 Benedict's
reagent is used as a simple test for reducing sugars. A reducing
sugar is a carbohydrate possessing either a free aldehyde or free ketone functional
group as part of its molecular structure. Recall from lectures that functional
groups are the regions of a molecule that gives it particular properties. A
single molecule can have more than one functional group as part of its structure.
When a molecule with multiple functional groups is involved in a reaction all,
some or none of the functional groups may be involved.
Benedict's
reagent is used as a simple test for reducing sugars. A reducing
sugar is a carbohydrate possessing either a free aldehyde or free ketone functional
group as part of its molecular structure. Recall from lectures that functional
groups are the regions of a molecule that gives it particular properties. A
single molecule can have more than one functional group as part of its structure.
When a molecule with multiple functional groups is involved in a reaction all,
some or none of the functional groups may be involved.
Glucose is a reducing sugar, while the disaccharide sucrose is
not. As a result, glucose heated in Benedict's reagent reduces Cu++ ions to
form a green to brick-red precipitate depending on the amount of sugar present.
In the lab you prepared 3 tubes:
1. Water and Benedict's reagent
2. Glucose and Benedict's reagent
3. Sucrose and Benedict's reagent
View
the three tubes
Consider the following questions. For answers to the questions consult your
lab manual, textbook and lecture notes.
- Which sugar is a reducing sugar?
- Why is one sugar a reducing sugar and the other not?
- Which functional group is responsible for the difference?
- What is the purpose of each tube?
- Why is the functional group not available in the non-reducing sugar?
Exercise 2 - Glucose Polymerization
Glucose & Glucose Polymers
Glucose is one of the most important biological carbohydrates. It is produced
by plants during photosynthesis and as such it is a common food source for non-autotrophs.
Glucose, once produced by the plant, or ingested by animals or fungi needs to
be stored for later use. There are two main glucose polymers used for storage:
starch by plants and glycogen by animals.
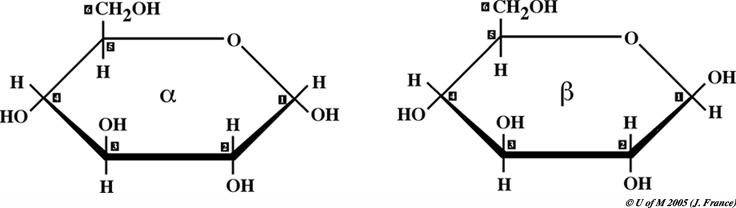
Examine
the glucose model from the front of your lab manual
Note the location of each of the six carbons.
Draw three glocose bonded by 1-4 bonds. It should look like the
image below:
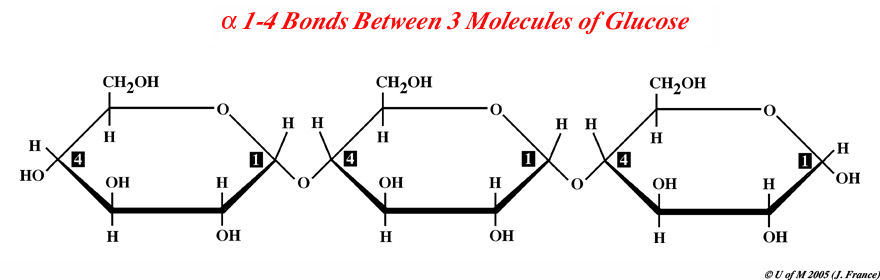
Keep in mind that a regular starch molecule found in a plant
storage site like a potato can be 100's of thousands on glucose units long.
Consider the following questions. For answers to the questions consult your
lab manual, textbook and lecture notes. If you are still unsure consult with
one of the instructors.
1. What molecule would be removed to make the connection (bond)?
2. What is this type of reaction called?
3. If you wanted to make cellulose, another glucose polymer, could you use
the same cut out or would need a different one?
4. Look at your glucose molecule. How many of the -OH groups can rotate freely?
Exercise 3 - Test for starch
Cut a small section of potato and place it in a petri dish. Place a drop of
IKI (iodine potassium iodide) solution on the surface of the potato. IKI is
a stain routinely used to locate starch deposits. A dark blue-black colour indicates
that starch is present.
View
the results of the test
Consider the following questions. For answers to the questions consult your
lab manual, textbook and lecture notes.
- Does the potato tissue contain starch?
- Why would the potato contain starch?
First published Sept 95: Modified June 2020
Copyright © Michael Shaw 2019 (Images and Text)
 Back
to Lab Index
Back
to Lab Index  Forward
to Next Page
Forward
to Next Page  University
of Manitoba Home
University
of Manitoba Home
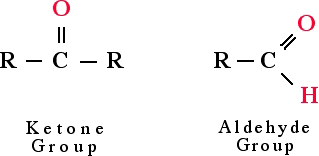 Benedict's
reagent is used as a simple test for reducing sugars. A reducing
sugar is a carbohydrate possessing either a free aldehyde or free ketone functional
group as part of its molecular structure. Recall from lectures that functional
groups are the regions of a molecule that gives it particular properties. A
single molecule can have more than one functional group as part of its structure.
When a molecule with multiple functional groups is involved in a reaction all,
some or none of the functional groups may be involved.
Benedict's
reagent is used as a simple test for reducing sugars. A reducing
sugar is a carbohydrate possessing either a free aldehyde or free ketone functional
group as part of its molecular structure. Recall from lectures that functional
groups are the regions of a molecule that gives it particular properties. A
single molecule can have more than one functional group as part of its structure.
When a molecule with multiple functional groups is involved in a reaction all,
some or none of the functional groups may be involved.
