© J. van Wijngaarden 2021
research
Probing the energy landscapes of molecules
The energies of molecular motions, such as rotation and vibration, are unique fingerprints of a compound. With support from modern compuational chemistry methods, we apply state-of-the-art ultrahigh resolution spectroscopic techniques to probe the lowest energy motions of molecules. Spectra recorded in the far- infrared region using synchrotron light from the Canadian Light Source provide measures of molecular vibrations with unprecedented detail while our custom- built microwave spectrometers at the University of Manitoba shed light on rotations and molecular re-arrangements. Our research fits under a number of diverse themes including (but not limited to):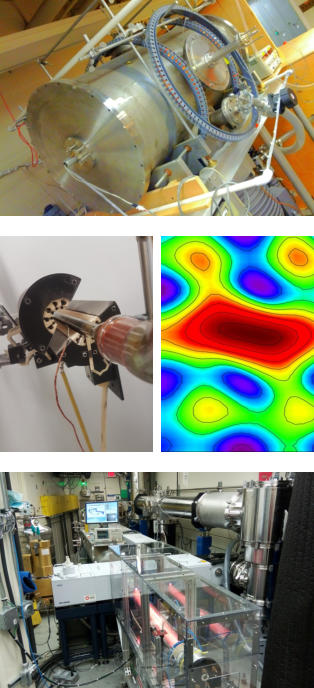
Driving forces of conformational equilibria
You probably remember from your introductory chemistry classes that molecules can adopt different geometries depending on their arrangement around single and double bonds, but did you know that each form has its own distinct rotational spectrum? For diallylamine , for example, we have shown that there are 42 possible geometries depending on the positions of the two side chains relative to the central NH functional group. We identified spectral fingerprints of the four lowest energy forms and found that the rich conformational mixture is a result of a careful balance of hyperconjugative and steric effects.Astrochemical signatures
Did you know that astronomers use microwave and infrared spectra to identify new species in space? We generate potential candidates in the lab such as HCCNCS and HCCCCNCS using high voltage electric discharge sources and record their microwave and infrared spectra for inclusion in astronomy catalogs. Each new detection in space provides another piece in the puzzle of the chemical evolution of the Universe.Accurate molecular geometries
Do you know how scientists find the bond lengths and angles of a molecule with great accuracy and precision? They use Molecular Rotational Resonance (MRR) spectroscopy, the electric field analog of NMR spectroscopy. We have two instruments in our lab that allow us to measure even the most subtle changes in molecular geometry. We can even show the preference for one resonance structure over another as in our studies on fluorinated rings of anisole and benzaldehyde .Microsolvation
Did you ever consider how the properties of a molecule change when solvent molecules pack around it? Our spectra help us identify the lowest energy binding sites for solvent, which conformers are more easily solvated and the dynamics of the solvent-solute interaction. In our study of the microsolvation of thiophene , for example, we identified two facile tunnelling motions involving the water hopping across the ring and rotating about its symmetry axis.










vW Research Lab
The University of Manitoba campuses are located on original lands of Anishinaabeg, Cree, Oji-Cree, Dakota, and Dene peoples, and on the homeland of the Metis Nation.
research
Probing the energy landscapes of molecules
The energies of molecular motions, such as rotation and vibration, are unique fingerprints of a compound. With support from modern compuational chemistry methods, we apply state-of-the-art ultrahigh resolution spectroscopic techniques to probe the lowest energy motions of molecules. Spectra recorded in the far-infrared region using synchrotron light from the Canadian Light Source provide measures of molecular vibrations with unprecedented detail while our custom-built microwave spectrometers at the University of Manitoba shed light on rotations and molecular re-arrangements. Our research fits under a number of diverse themes including (but not limited to):
Driving forces of conformational equilibria
You probably remember from your introductory chemistry classes that molecules can adopt different geometries depending on their arrangement around single and double bonds, but did you know that each form has its own distinct rotational spectrum? For diallylamine , for example, we have shown that there are 42 possible geometries depending on the positions of the two side chains relative to the central NH functional group. We identified spectral fingerprints of the four lowest energy forms and found that the rich conformational mixture is a result of a careful balance of hyperconjugative and steric effects.Astrochemical signatures
Did you know that astronomers use microwave and infrared spectra to identify new species in space? We generate potential candidates in the lab such as HCCNCS and HCCCCNCS using high voltage electric discharge sources and record their microwave and infrared spectra for inclusion in astronomy catalogs. Each new detection in space provides another piece in the puzzle of the chemical evolution of the Universe.Accurate molecular geometries
Do you know how scientists find the bond lengths and angles of a molecule with great accuracy and precision? They use Molecular Rotational Resonance (MRR) spectroscopy, the electric field analog of NMR spectroscopy. We have two instruments in our lab that allow us to measure even the most subtle changes in molecular geometry. We can even show the preference for one resonance structure over another as in our studies on fluorinated rings of anisole and benzaldehyde .Microsolvation
Did you ever consider how the properties of a molecule change when solvent molecules pack around it? Our spectra help us identify the lowest energy binding sites for solvent, which conformers are more easily solvated and the dynamics of the solvent-solute interaction. In our study of the microsolvation of thiophene , for example, we identified two facile tunnelling motions involving the water hopping across the ring and rotating about its symmetry axis.





vW Researh Lab