Key Types of Bonding in Chemistry

Course Menu
Covalent Bonds
Covalent Bonding involves the sharing of an electron to help satisfy the completion of each elements valence shell. It is a strong type of chemical bond which two atoms share one or more pairs of valence electrons. This is demonstrated below:
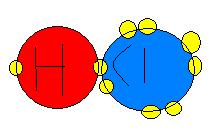
Where Cl=Clorine and H=Hydrogen. The yellow circles represent the electrons, and as you can see Clorine and Hydrogen are sharing 1 electron. This Satisfies both of the elements outer valence as they are at their max capacity for electrons. There are no charges associated with this type of bonding and there is no 1 atom that is more electronegative than the other. If this were the case it would be a Polar Covalent Bond. Note Polar Covalent Bonds usually do not occur with Clorine.
If you have further questions and would like to discuss the problems that you are having feel free to contact me at:
Melanie Lalonde
123 Fun Street
Winnipeg,MB, T3C4C6
123 Fun Street
Winnipeg,MB, T3C4C6
or if it is more convient you can always e-mail!
*copyright 2009 by Melanie Lalonde* *Internet Explorer V 8*