Key Types of Bonding in Chemistry

Course Menu
Hydrogen Bonding
Hydrogen bonding is a type of weak chemical bond that is formed when the slightly positive hydrogen atom of a polar covalent bond in one molecule is attracted the slightly negative atom of a polar covalent bond in another molecule. This is demonstrated below with the most commonly bonded atoms.
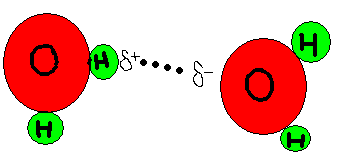
O is representative of a Oxygen atom and H is a Hydrogen atom. The Oxygen atom is electronegative and is the slightly negative atom and pulls the slightly positive Hydrogen atom from the other water molecule close to it but does not exchange or share electrons with one another.
If you have further questions and would like to discuss the problems that you are having feel free to contact me at:
Melanie Lalonde123 Fun Street
Winnipeg,MB, T3C4C6
or if it is more convient you can always e-mail!
*copyright 2009 by Melanie Lalonde* *Internet Explorer V 8*